Energy From Nuclear Fission
advertisement
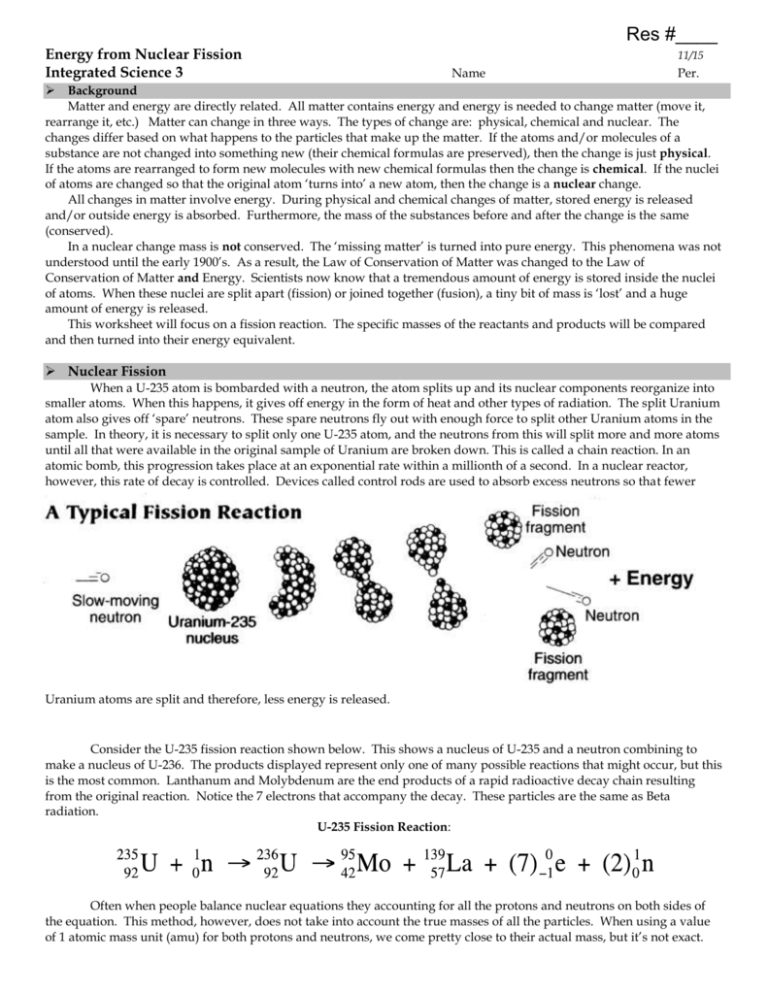
Res #____ Energy from Nuclear Fission Integrated Science 3 11/15 Name Per. Background Matter and energy are directly related. All matter contains energy and energy is needed to change matter (move it, rearrange it, etc.) Matter can change in three ways. The types of change are: physical, chemical and nuclear. The changes differ based on what happens to the particles that make up the matter. If the atoms and/or molecules of a substance are not changed into something new (their chemical formulas are preserved), then the change is just physical. If the atoms are rearranged to form new molecules with new chemical formulas then the change is chemical. If the nuclei of atoms are changed so that the original atom ‘turns into’ a new atom, then the change is a nuclear change. All changes in matter involve energy. During physical and chemical changes of matter, stored energy is released and/or outside energy is absorbed. Furthermore, the mass of the substances before and after the change is the same (conserved). In a nuclear change mass is not conserved. The ‘missing matter’ is turned into pure energy. This phenomena was not understood until the early 1900’s. As a result, the Law of Conservation of Matter was changed to the Law of Conservation of Matter and Energy. Scientists now know that a tremendous amount of energy is stored inside the nuclei of atoms. When these nuclei are split apart (fission) or joined together (fusion), a tiny bit of mass is ‘lost’ and a huge amount of energy is released. This worksheet will focus on a fission reaction. The specific masses of the reactants and products will be compared and then turned into their energy equivalent. Nuclear Fission When a U-235 atom is bombarded with a neutron, the atom splits up and its nuclear components reorganize into smaller atoms. When this happens, it gives off energy in the form of heat and other types of radiation. The split Uranium atom also gives off ‘spare’ neutrons. These spare neutrons fly out with enough force to split other Uranium atoms in the sample. In theory, it is necessary to split only one U-235 atom, and the neutrons from this will split more and more atoms until all that were available in the original sample of Uranium are broken down. This is called a chain reaction. In an atomic bomb, this progression takes place at an exponential rate within a millionth of a second. In a nuclear reactor, however, this rate of decay is controlled. Devices called control rods are used to absorb excess neutrons so that fewer Uranium atoms are split and therefore, less energy is released. Consider the U-235 fission reaction shown below. This shows a nucleus of U-235 and a neutron combining to make a nucleus of U-236. The products displayed represent only one of many possible reactions that might occur, but this is the most common. Lanthanum and Molybdenum are the end products of a rapid radioactive decay chain resulting from the original reaction. Notice the 7 electrons that accompany the decay. These particles are the same as Beta radiation. U-235 Fission Reaction: Often when people balance nuclear equations they accounting for all the protons and neutrons on both sides of the equation. This method, however, does not take into account the true masses of all the particles. When using a value of 1 atomic mass unit (amu) for both protons and neutrons, we come pretty close to their actual mass, but it’s not exact. One amu is actually 1.6604 X 10-24 grams. Knowing this value will allow us to study the mass of substances before and after a nuclear reaction in more depth. Procedures The following tables list the masses of the individual particles found in the U-235 fission reaction described on the front page. Use them to complete the following set of procedures. 1. Add up the mass of the reactants and products. DO NOT ROUND. Mass of Reactants Reactants U-235 nucleus one neutron Total Mass of Products Mass (amu) 235.0439 1.0087 Products molybdenum nucleus lanthanum nucleus 7 electrons 2 neutrons Total Mass (amu) 94.9058 138.9061 0.0039 2.0174 2. Calculate the difference between the mass of the reactants and the products. Show all of your work. 3. Convert the amu value difference to grams knowing that 1 amu = 1.6604 X 10-24 grams. Round all answers to 4 decimal places. 4. Explain why this difference in mass occurs. Hint: think about the Law of Conservation of Mass and Energy. 5. Consider Einstein's Equation E = mc2. Ultimately, the world's most famous equation demonstrates that at an extremely high speed, matter and energy are interchangeable. In other words, the amount of potential energy (joules) in a given amount of mass (kilograms) can be determined. In the equation, (c) is the speed of light or 2.998 X 108 meters per second. Convert the mass determined in step 3 to energy in joules (J). First, convert grams (g) to kilograms (kg). Then use the following equation: (energy in J) = (mass in kg) X (speed of light in meters per second)2 Round your answer to 4 decimal places and include units with your answer. 6. Remember that the value you just calculated represents the energy (in joules) evolved due to the conversion of mass to energy from a single atomic fission reaction (with one U-235). But in a gram of U-235, there are 2.6 X 1022 atoms. Calculate the amount of energy evolved if an entire gram of U-235 underwent fission. 7. The fission process is very inefficient. Only 10% (0.10) of the atoms of U-235 are actually split by the nuclear chain reaction. Given this inefficiency, what is the actual energy yield of a gram of U-235? Where does the energy go? Energy is released from fission reactions as: 84 % is kinetic energy of fission products, 2.5 % is kinetic energy of neutrons, 2.5 % is instantaneous release of gamma rays, and 11.0 % is gradual radioactive decay of fission products. Since anything in motion has heat energy, all forms of radiation actually release heat (which is what is used to generate electricity). Note that, per particle, about 7 times as much energy is released in nuclear fusion (when hydrogen atoms fuse) than in fission.