Data supplement - Journal of The Royal Society Interface

Cerebrospinal Fluid Dynamics in the Human Cranial Subarachnoid Space – An Overlooked
Mediator of Cerebral Disease. Part I: Computational Model
Cerebrospinal Fluid Dynamics in the Human Cranial Subarachnoid Space –
An Overlooked Mediator of Cerebral Disease. Part I: Computational Model
Sumeet Gupta, PhD 1 , Michaela Soellinger, PhD 2,3 , Deborah M. Gryzibowski,
PhD 4 , Peter Boesiger, PhD 3 , John Biddiscombe, BEng 5 , Dimos Poulikakos,
PhD 1 , Vartan Kurtcuoglu, PhD 1,*
Data Supplement
Details on the spatial and temporal discretization of the governing equations
The governing equations were discretized using a second order upwind scheme in space and second order implicit scheme in time (Warming & Beam 1976).
Pressure-velocity coupling was achieved with the SIMPLEC algorithm
(Vandoormaal & Raithby 1984). The gradients of velocity and pressure were calculated using the Green-Gauss node based gradient evaluation scheme
(Rauch et al. 1991). This scheme reconstructs exact values of a linear function at a node from surrounding cell-centered values on meshes by solving a constrained minimization problem, preserving a second order spatial accuracy.
For the CFD calculations, the convergence criterion was set to 10 -4 for the maximum residual.
We constructed the computational grid using Ansys ICEM CFD (Ansys Inc.,
Canonsburg, PA, USA). We divided the treated domain into three regions based on the characteristic sizes of the present geometric features and then performed the grid generation on each region separately, using non-conformal interfaces to connect them. For laminar flows such as that of CSF in the SAS, the maximum
Cerebrospinal Fluid Dynamics in the Human Cranial Subarachnoid Space – An Overlooked
Mediator of Cerebral Disease. Part I: Computational Model velocity gradients occur in the boundary layers. It is distinctly necessary to capture these gradients in order to predict accurate flow patterns. For this purpose, we discretized the grid near the domain walls was using prism-shaped grid cells inflating orthogonally out of the wall into the fluid domain. The height of the inflation layer was approximately determined using the laminar scaling law
Re
L
1/ 2 , where δ is the boundary layer thickness, L is the region-dependent characteristic length and Re
L
the Reynolds number. The central inviscid region was discretized with tetrahedra. In order to ensure grid independence of the solution, the computations were carried out using three levels of successively refined computational grids consisting of approximately 10 million, 14 million and
19 million cells, respectively.
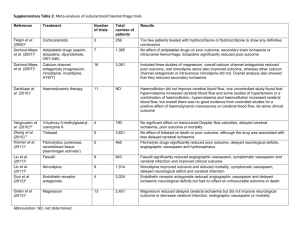
Figure 7a shows differences in pressure between the three computational grids along a line in the subarachnoid space that was identified as being particularly sensitive to variations in both the temporal and spatial discretization. The maximum pressure difference between the coarse and the medium size mesh is less than 3%. There is very little difference (< 1%) between the results calculated with the fine and the medium meshes, which is why the medium size mesh was used for the present computations. Figures 7b and 7c show the period and temporal step size independence of the solution. The results indicate that at least four periods with 1600 time steps per period are necessary to reach results independent of temporal discretization.
Sensitivity of the reported results to the MRI-acquired boundary conditions
The accuracy of the here employed phase contrast MRI for flow motion analysis has been evaluated before, indicating a relative error of +-10% in the peak velocity (Lingamneni et al. 1995). In order to keep this error as low as possible in our current work, we applied a first order concomitant field correction (Bernstein
2
Cerebrospinal Fluid Dynamics in the Human Cranial Subarachnoid Space – An Overlooked
Mediator of Cerebral Disease. Part I: Computational Model et al. 1998) and a linear background phase subtraction. At the edges of the boundary, we impose zero velocity relative to the domain wall and expect no noteworthy error there. Between the peak velocity locations in the center area of the boundary and zero relative velocity at the wall, the relative error is likely to exceed 10%, but the actual value of the error is unknown (Hoogeveen et al.
1999). If we assume that there is an average relative error of 15% over the entire flow profile at the respective boundary, this will translate to a relative error of again 15% in the CSF stroke volume due to the incompressibility of the fluid, but not necessary to the same error in velocity at every location in the domain. Yet because of the low Reynolds numbers in the entire domain, the flow is almost certain to remain in the laminar regime, even if the velocity field is underestimated by as much as 5o%. Consequently, we do not expect a significant qualitative difference between the calculated and the actual flow field, but do anticipate quantitative variations whose spatial distribution cannot be quantified. A more detailed discussion of the accuracy of MRI acquired boundary conditions in the CSF space can be found in (Soellinger 2008).
References
Bernstein, M. A., Zhou, X. J., Polzin, J. A., King, K. F., Ganin, A., Pelc, N. J. & Glover,
G. H. 1998 Concomitant gradient terms in phase contrast MR: analysis and correction. Magn Reson Med 39 , 300-8.
Hoogeveen, R. M., Bakker, C. J. & Viergever, M. A. 1999 MR phase-contrast flow measurement with limited spatial resolution in small vessels: value of modelbased image analysis. Magn Reson Med 41 , 520-8.
Lingamneni, A., Hardy, P. A., Powell, K. A., Pelc, N. J. & White, R. D. 1995 Validation of cine phase-contrast MR imaging for motion analysis. J Magn Reson Imaging 5 ,
331-8.
Rauch, R. D., Batira, J. T. & Yang, N. T. Y. 1991 Spatial adaption procedures on unstructured meshes for accurate unsteady aerodynamic flow computations. AIAA
Technical Report , AIAA-91-1106.
Soellinger, M. 2008 Assessment of intracranial dynamics using MRI. Zurich,
Switzerland: ETH Zurich Dissertaton Nr. 17596.
3
Cerebrospinal Fluid Dynamics in the Human Cranial Subarachnoid Space – An Overlooked
Mediator of Cerebral Disease. Part I: Computational Model
Vandoormaal, J. P. & Raithby, G. D. 1984 Enhancements of the SIMPLE method for predicting incompressible fluid-flows. Numer Heat Transfer 7 , 147-163.
Warming, R. F. & Beam, R. M. 1976 Upwind 2nd-order difference schemes and applications in aerodynamic flows. AIAA J 14 , 1241-1249.
4
Cerebrospinal Fluid Dynamics in the Human Cranial Subarachnoid Space – An Overlooked
Mediator of Cerebral Disease. Part I: Computational Model
Figure Captions
Figure 7: Results of temporal and spatial discretization independence studies along a critical path shown in d): Pressure along this path as calculated with a) different meshes, b) for several periods, c) with different number of time steps per period.
5