Supplementary Methods - Word file (41 KB )
advertisement

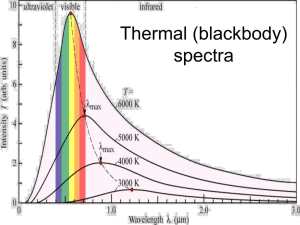
Supplementary material Study area and populations 300 nest boxes have been installed in deciduous forests in the surroundings of the University of Lausanne (Switzerland) where we have breeding populations of Starlings and Great tits. The nest boxes are made of an internal wooden box with a thick and opaque plastic cover (Heeb et al. 2003). From early March 2003 onwards, the breeding activity of the birds was followed every day from nest building onwards. For each breeding attempt, the date of laying of the first egg, clutch size and date of hatching was recorded. The growth of the nestlings was followed from hatching to fledging from the nest. A second starling population breeding in southern England was used to provide independent measurements of reflectance spectra and nest irradiance. Measures of flange and skin reflectance For both species in Switzerland, reflectance spectra (280 – 700 nm) were recorded on six day old nestlings using an S2000 spectrophotometer, with a DH-2000 deuterium-halogen light source and 2.5 mm diameter coaxial reflectance probe, and the OOIBase32 TM spectrophotometer operating software (Ocean Optics, Inc., Dunedin, FL, USA). Gapes were gently held open while the probe was held perpendicularly at a fixed distance to a tangent to the surface of the flange. The strobe illuminated an area of about 1.5 mm in diameter. The reflectance was calculated relative to a 99% white Spectralon reflection standard sampled every eight scans. For each Starling nestling we collected a total of eight spectra on the upper and lower flanges without any treatment and eight spectra with either +UV or UV treatment (see below). We also measured one spectrum of the skin of the back of the body, one spectrum where the legs connect to the body and one spectrum on the forehead between the eyes (we measured each of these spectra again for the +UV and UV treatments). The number of spectra measured for the curves shown in Fig. 1ab are: natural flanges: 21 individuals, 168 spectra; flanges –UV: 12 individuals, 92 spectra; flanges + UV: 9 individuals, 69 spectra; skin natural: 21 individuals, 54 spectra; skin –UV: 10 individuals, 29 spectra; skin +UV: 7 individuals, 20 spectra. Spectra obtained from nestlings in the English Starling population were measured by using an Ocean optics PX-2 light source and by holding the probe onto the sample. The irradiance spectra in the nest environment was measured in 5 nests (4 spectra per nest, Fig 1e). Spectra for nestling flanges were measured in 5 nests (n=10 chicks, 4 spectra per chicks aged 3-5 days old). Body skin of chicks was measured on the back of head, body back and throat (n=3 nests, n=6 chicks, 4 spectra per chick per region). Methods used for measurement of these spectra and irradiance are reported in Hunt et al (2003). For Great tits, we measured for each nestling a total of four spectra of the upper and lower flanges without any treatment and four spectra with either +UV or UV treatment, (Fig 1cd). We measured one spectrum of the throat skin and one spectrum of the skin above a hind leg with no treatment (flanges: n = 25 individuals, 109 spectra; body skin: n = 22, 39 spectra); +UV (n = 10, 42 spectra); -UV (n = 10, 42 spectra). For the Swiss data we calculated the median of the spectra values and the standard deviation every 20 m. During the measurements we removed nestling from the nest and kept them in warmed boxes. We always made sure that at least one or two nestlings remained in the nest. Before replacing the young back in the nest they were fed ad libitum with pieces of mealworms. Calculation of the reflectance peaks on the skin We measured immune responses in a number of Starling nestlings and calculated the median of their spectra of flanges and body skin. From this median spectra we obtained for flanges and body skin the median reflectance values between 320 and 360 nm (M1), between 440 and 480 nm (M2) and between 540 and 700 nm (M3). M1 represents the median maximal value of UV reflectance, M2 gives the median baseline reflectance value, and M3 stands for the median reflectance in the visible spectrum. For each nestling peak flange reflectance, illustrating spectral brightness was obtained as (M1 – M2) + (M3 – M2). To this value we added (M1 – M3) for the body skin and used the final sum to examine the relationship between brightness of skin reflectance and morphological traits (see below). Modifications of flange and skin reflectance We carried out one experiment with six days old Starling nestlings from the second broods. We removed the young from the nest and had them defecate before measuring their body mass to the nearest 0.1 g with a portable "Sartorius" electronic balance. After two hours of experiment we measured again body mass of nestlings. Within each brood, we randomly assigned nestlings in a group that reflected in the UV (+UV) and in a second group where flange and body skin UV reflectance was specifically reduced (-UV). Individuals were individually marked by plucking down feathers on the head and wings and ranked according to their relative mass within the brood. After ranking the nestlings in relation to their body mass, they were assigned alternatively to one of the two treatments (+UV or -UV) in each brood order to obtain similar overall mean body masses between treatments. The order of the treatments was randomized between broods, thus ensuring that heaviest nestlings appeared as often in the two treatments. In this experiment the body and mouth flanges were covered with a small quantity of petroleum jelly (+UV) or petroleum jelly with UV protection (-UV). A total of 40 nestlings in 12 broods (21 in -UV and 19 in +UV), were tested in this experiment. Nestlings in the two groups did not differ in their body mass before the experiment (t-test: t = 0.1, DF = 38, P = 0.92) and they significantly gained mass during the experiment (mass gain: 1.05 ± 0.19 (g ± se), n = 40; t-test = 5.61, P = 0.0005). For the +UV treatment, we spread a small quantity of petroleum jelly (Petroleum: 93.95%, Cetiol B: 6%, BHT: 0.05%) with a cotton swab on both inner and outer sides of the flanges and on the whole of their body. For the -UV treatment we blocked UV reflectance by spreading on the body and mouth flanges petroleum jelly mixed with UV protection (Petroleum jelly: 79.95%, Cetiol B: 6%, BHT: 0.05%, Parsol 1789: 3%, Parsol MCX: 6%, Eusolex OS: 5%; Roche). In both treatments, the excess product was gently removed from the skin with a clean paper tissue. Spectra in Figures 1a-c show that our -UV treatment reduced skin reflectance between 280 and 400 nm whilst the +UV treatment allowed for reflectance in these wavelengths. UV blocking substances used by Roche have been chosen for their neutral effect on vertebrate skin. However, any potential effect of petroleum jelly applied on the skin on nestling begging performance is unknown. An examination of spectra for a number of nestlings revealed that after 180 minutes, the differences between nestlings in the -UV and +UV treatments were still present on the flanges. However, the body skin had reduced UV reflectance presumably due to close body contact between nestlings in the brood, and between nestlings and the nest. We performed a second experiment when Starling nestlings of the first brood and Great tit broods were six days old. In this experiment petroleum jelly was only applied to mouth flanges. We followed the same procedure as described above with a difference in that the larger brood sizes in Great tits allowed us to assign young in a third treatment where the young were handled in the same way but where petroleum jelly was not applied and thus acted as a second control. A total of 103 Starling nestlings from 27 broods and 333 Great tits in 53 broods were included in this experiment. There were no significant differences in initial body mass between Starlings in the two treatments (F1,101 = 0.04, P = 0.84). Nestlings significantly gained mass during the two hour interval of the experiment (mean mass gain: 1.33 ± 0.17 (g ± se), n = 103 nestlings; t-test = 7.93, P < 0.0001). In Great tits, there were no significant differences in initial body mass between nestlings in the three treatments (F2,330 = 0.084, P = 0.92). Nestlings significantly gained mass during the two hour interval of the experiment (mass gain: 0.29 ± 0.016, n = 333 nestlings; t-test = 17.7, P < 0.0001). In all experiments, immediately after applying the petroleum jelly, all the young were replaced in the nests for 120 2 minutes. After this time interval, the young were taken out of the nest and weighed again to the nearest 0.1 g in order to determine individual changes in body mass (see below). Assessment of nestling immunocompetence The immune response of 14 day old Starling nestlings towards phytohaemaglutinin (PHA) was tested. Nestlings were subcutaneously injected in the right wing web with 0.1 mg of PHA dissolved in 0.02ml of phosphate-buffered saline (PBS). The thickness of the wing web was measured at the injection site with a pressure-sensitive micrometer (Mitutoyo model 2046F, accuracy 0.01 mm) before and 24 h (± 1h) after the injection. A control injection of PBS in the left wing was not done as this has previously been shown to be unnecessary (Smits et al. 1999). We estimated the T-cell-mediated immune response or wing web index as the change in thickness of the right wing web (Brinkhof et al. 1999). Nestling immune response was not significantly correlated with their body mass (F1,13= 1.22, P = 0.29) or with their tarsus length (F1,13= 1.47, P = 0.25). Statistical analyses For each brood, we calculated the mean mass gained by nestlings in the brood (sum of all individual masses gained divided by brood size). We then calculated for each nestling the difference in mass gain from the brood mean mass gain. These calculations allowed us to correct for inter-brood variation in total brood mass gained and use the value of each young as a data point in a population of mass deviations around a mean of zero mass gains (for the three experiments P > 0.60). In the analyses we included the effect of nest as a random effect and present the effect of treatment as a factor on individual relative mass gain. All analyses were made with JMP (Sall & Lehmann 1996). Ethical note The experiments were carried out according to local regulations and had no detectable long term effect on the nestlings. We found no significant difference in mass difference between days 6 and 14 in nestlings in the different treatments. References Brinkhof, M.G.W., Heeb, P., Kölliker, M., & Richner, H. 1999. Immunocompetence of nestling great tits in relation to rearing environment and parentage. Proc. Roy. Soc. Lond. 266, 2315 – 2322. Sall, J. & Lehman, A. 1996. JMP Start Statistics. A guide to statistics and data analysis using JMP and JMP IN software. Belmont, California: Duxbury Press. Smith, J. E. et al. 1999. Simplifying the phytohemaglutinin skin testing technique in studies of avian immunocompetence. Funct. Ecol. 13: 567-577.