Microsoft Word Format - University of Toronto
advertisement

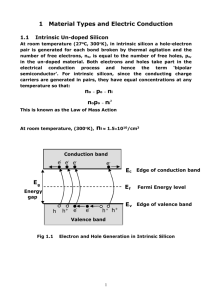
ADVANCED UNDERGRADUATE LABORATORY HALL Semiconductor Resistance, Band Gap, and Hall Effect Revisions: November 2011, January 2016: David Bailey October 2010: Henry van Driel February 2006: Jason Harlow November 1996: David Bailey March 1990: John Pitre & Taek-Soon Yoon Copyright © 2011-2016 University of Toronto This work is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. (http://creativecommons.org/licenses/by-nc-sa/3.0/) Introduction Solid materials are usually classified as metals, semiconductors, or insulators. In general, metals conduct electricity better than semiconductors, and insulators essentially do not conduct electricity at all, but the critical distinguishing characteristic between the 3 classes of materials is their electronic energy structure. The basic parameters determining the conductivity of a semiconductor are the band gap, the charge carrier densities (intrinsic and extrinsic), and the mobility of the charge carriers. The goal of this experiment is to measure these parameters for two doped germanium samples. The allowed energies of electrons inside solid materials are quantum mechanically restricted to certain ranges known as energy bands. For semiconductors the lower energy bands are completely filled in intrinsic semiconductors at low temperatures and are known as valence bands; the first band that is normally empty at low temperatures is known as the conduction band. The difference in energy between the top of the valence band and the bottom of the conduction band is known as the fundamental energy gap. With an increase in temperature electrons can be thermally activated across the relatively small band gap of a semiconductor allowing the free carriers to move. Alternatively, doping can add free carriers to the conduction or valence band. Materials in which an energy band is always partially filled are metals, with the partially filled bands allows electrons to move freely. Insulators are materials where the band gap is so large that the conduction band is essentially always empty. As a result, the resistivity of an insulator is very high. The resistance of a semiconductor is normally several orders of magnitude higher than that of a metal, but it is the temperature dependence of the resistance that distinguishes a metal from a semiconductor. The resistance of the semiconductor germanium (Ge) near room temperature decreases with increasing temperature, whereas in a metal the resistance generally increases with temperature. In a metal the number of carriers is fixed, and the increase in resistance of the metal is because of the increased number of thermally excited lattice vibrations (phonons) from which the charge carriers can scatter. In a semiconductor the increase in scattering is usually overwhelmed by the exponential increase in the number of carriers, as a result of thermal excitation across the energy gap. The temperature dependence of the resistance can be used to determine the band gap. The Hall voltage is the voltage transverse to both magnetic field and current. It appears when a magnetic field transverse to the direction of current flow is applied. The sign of the Hall voltage determines whether the dominant carriers in the semiconductor are electrons or holes; its magnitude is a measure of the carrier concentration. Theory Semiconductors, metals, electrolytes and other conducting materials have charge carriers that are free to move about in the substance, not being tightly bound to any particular atom or molecule. If an electric field exists in such a conducting material, positive and/or negative charge carriers tend to drift towards the electrode or contact of opposite polarity, thus causing an electric current. The charge carriers are not able to travel unimpeded from one contact to another; in a solid they collide with defects in the lattice structure such as impurity atoms and lattice vibrations, and in a liquid or gas with other ions, molecules, or atoms. These collisions impede the current flow and give rise to electrical resistance. 2 Conductivity To explain the conduction processes in a semiconductor we need to assume that the electric current may be caused by the flow of two types of charged carriers, electrons (in conduction band) and holes (absence of an electron from valence band). The hole may be looked upon as a particle carrying a positive electronic charge and its movement is analogous to that of the gap in a line of cars as they accelerate away from a traffic light which has just turned green: as the cars (electrons) move forward in turn, the gap (hole) moves in the opposite direction. The electrical conductivity may be expressed in terms of the number of charge carriers, their charge, and their mobility; the average velocity of the carrier per unit of electric field is s = pemh - n(-e)me = pemh + neme (1) where p is the number density of holes in m-3, n is the number density of electrons in m-3, e=1.602×10-19 C is the magnitude of the charge of the electron, h is the hole mobility in m2s-1V-1, and e is the electron mobility in m2s-1V-1. Equation (1) applies to a semiconductor when the numbers of electrons and holes present are both significant. If the semiconductor is “doped” by introducing suitable impurities, the charge carriers can be made predominantly of one type. In germanium, which is in Group 4 of the periodic table, a Group 5 impurity such as antimony has an extra electron which becomes a free charge carrier and the semiconductor is then said to be n-type. Conversely, a Group 3 impurity such as indium makes germanium p-type, since the missing electron is equivalent to a hole in the valence band. Equation (1) thus becomes s = pemh (2) for a p-type semiconductor or s = neme (3) for an n-type semiconductor. In a pure semiconductor the number density n or p of charge carriers per unit volume is far smaller than in a metal. For example, in pure Ge at room temperature, n ≈ p ≈ 1018 m-3, whereas in a typical metal, n ≈ 1028 m-3. This results in a much larger conductivity in a metal than in a semiconductor. However as the temperature is raised in a semiconductor the conductivity increases rapidly, whereas in metal the conductivity decreases slowly. This striking difference in behaviour is a direct consequence of the property of a semiconductor which distinguishes it from a metal, namely, the existence of a relatively small energy gap between electron states in the valence band (which correspond to the bound electrons constituting the covalent bonds between the atoms) and electron states in the conduction band (which correspond to electrons free to conduct electricity). At low temperatures, all the electrons are in the valence band which is completely full (corresponding to the saturation of the 4 covalent bonds by the 8 electrons available from each Ge atom of valence 4 and its 4 nearest neighbours), so that there are no free charges or carriers, except those induced by impurities. Such a semiconductor is then referred to as extrinsic. As the temperature, T, is raised, the semiconductor becomes “intrinsic”, meaning that the number density of charge carriers, as determined by the band populations of the atoms, determines the resistivity. In an intrinsic semiconductor the number of electrons is equal to the number of holes, because the thermal excitation of an electron leaves behind a hole in the valence band. It can be shown that: 3 (4) where the subscript i denotes “intrinsic”, T is the temperature in Kelvin, ΔE is the band gap energy, and kB=1.38×10-23 J/K is the Boltzmann’s constant. The T3/2 term is a phase space factor; from the Uncertainty principle, the density of momentum states per unit volume dN /dV µ (2 p / relativistic electrons/holes in a semiconductor: ( ) ) 3, so for non- 3 2 2mE dN m3 / 2 E 3 / 2 (5) µ ~ 3 3 dV where m is the effective mass of the electrons/holes (which, because of interactions with the lattice, can be very different from the rest mass of the electron). For a full derivation of equation (4), see Kittel’s “Introduction to Solid State Physics”, Chapter 8. At high temperatures, the temperature dependence of the conductivity is largely determined by the exponential term in equation (4), since the mobilities in equation (1) vary only as a power law of the temperature [see Adler (1964)], i.e. =CTB, where B and C are constants. So we expect the conductivity to be of the form (6) Hall Effect Consider the sample of p-type semiconductor with current density Jx flowing in the x-direction. In the presence of a magnetic field B0 along the z-direction, the holes will experience a force (the Lorentz force) driving them towards the bottom of the sample as shown in Figure 1. Figure 1: The Hall effect for positive charge carriers. This net charge displacement results in the bottom surface becoming positively charged, and the top surface becoming negatively charged. This process obviously cannot continue indefinitely since it would generate an infinite field in the y-direction. What happens is that the electric field Ey adjusts itself so that the force on the holes due to this field exactly cancels the Lorentz force: eE y = ev x Bz (7) 4 where vx is the hole velocity in m/s, and Bz is the magnetic field in Tesla. Now the current density in the x-direction is: Jx = epv x (8) so that Ey = J x Bz ep (9) we define the Hall coefficient as: RH = Ey Jx Bz = 1 ep (10) for p-type semiconductors. In a similar manner it can be shown that for an n-type semiconductor, in which the charge carriers are electrons with charge -e, the Hall coefficient is 1 1 =(11) -en en Note that the Hall coefficient has opposite signs for n and p-type semiconductors. The carrier concentration p or n can be determined from equations (10) or (11), and when combined in equation (2) or (3) with the measured conductivity, the carrier mobility h or e can be determined. RH = In any real semiconductor, both holes and electrons contribute to the conductivity. If one type of carrier is dominant, it is called the majority carrier and the other type is referred to as the minority carrier. The contribution to the conductivity from minority carriers cannot always be neglected, and may need to be included in your analysis. Safety Reminders Do not touch any part of the sample or sample card when the current is turned on; be aware that the sample may remain hot for some time after the heater is turned off. Do not touch the electromagnet connectors when the electromagnetic power supply is on. Keep metallic or electronic objects away from the magnets. It is not always obvious which objects might be forcefully attracted, e.g. all current Canadian coins are ferromagnetic. Also keep in mind that strong magnetic fields may erase magnetic strips on credit, bank or other similar cards, or damage electronic devices. NOTE: This is not a complete list of all hazards; we cannot warn against every possible dangerous stupidity, e.g. opening plugged-in electrical equipment, juggling cryostats, …. Experimenters must constantly use common sense to assess and avoid risks, e.g. if you spill liquid on the floor it will become slippery, sharp edges may cut you, …. If you are unsure whether something is safe, ask the supervising professor, the lab technologist, or the lab coordinator. If an accident or incident happens, you must let us know. More safety information is available at http://www.ehs.utoronto.ca/resources.htm. Experiment Measurements are made using the van der Pauw method described at http://www.nist.gov/pml/div683/hall_resistivity.cfm, using a square semiconductor sample with leads 5 attached at each corner. By flowing current through two leads, and measuring the voltage across the other two, at different temperatures and in different magnetic fields, the resistivity (ρ), Hall coefficient (RH), Hall mobility (h and e.), majority carrier sign (i.e. electrons or holes), carrier density (n or p), and band-gap of the sample can be determined. Hall Coefficient Following the NIST procedures described starting at http://www.nist.gov/pml/div683/hall_resistivity.cfm#hall, measure the Hall Coefficient, RH, of both nand p-doped samples at room temperature. Do not exceed the maximum current recommended by the Advanced Physics Lab technologists. One can use either a permanent magnet or an electromagnet for these measurements. It may also be interesting to measure the Hall coefficient of an undoped sample. Measure the magnetic field with a gaussmeter and determine the carrier densities. Conductivity Versus Temperature Following the NIST procedures described starting at http://www.nist.gov/pml/div683/hall_resistivity.cfm#resistivity, determine the variation of the conductivity with temperature over the range from room temperature to > 100 C. The interesting parts of the conductivity versus temperature curve occur below about 120°C. Overheating the sample may damage the Hall Probe! • • • • • Indicate the ranges of temperature over which the samples exhibit the characteristics of intrinsic and extrinsic conductivity. Is equation (4) consistent with the data over any temperature region? Is equation (6) consistent with the data? Determine the energy gap for both the n- and p- doped samples. Are the gaps all the same? It may be interesting to also determine the gap for an undoped sample. Can you determine any other interesting parameter? e.g. the mobility temperature exponent B, or the mean electron/hole effective mass (memp)1/2. Conductivity depends on both mobility and carrier density, while the Hall coefficient depends just on the carrier density, so measuring both the Hall coefficient and the conductivity as a function of temperature may allow you to determine the temperature dependence of the mobility and carrier density separately. 6 Bibliography R. B. Adler et al., Introduction to Semiconductor Physics, Wiley, 1964 (QC 612 S4A4). C. Kittel, Introduction to Solid State Physics, Wiley, 6th Edition, 1986 (QC 171 K5). w 7



![Semiconductor Theory and LEDs []](http://s2.studylib.net/store/data/005344282_1-002e940341a06a118163153cc1e4e06f-300x300.png)