Comparison of TCBQ and BN as solvents (one figure, one table).
advertisement

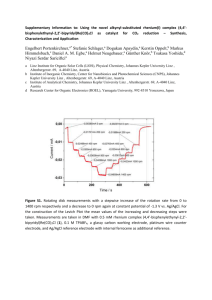
This journal is © the Owner Societies 2004 Electronic supplementary information for Physical Chemistry Chemical Physics Voltammetry of Immobilised Microdroplets Containing p-Chloranil on Basal Plane Pyrolytic Graphite Electrodes Debora Giovanelli1, Trevor J. Davies1, Li Jiang2, Timothy G. J. Jones2 and Richard G. Compton1* Supporting Information 1 Physical and Theoretical Chemistry Laboratory, University of Oxford, South Parks Road, Oxford, OX1 3QZ, United Kingdom. *To whom correspondence should be addressed: Email: richard.compton@chemistry.oxford.ac.uk Tel: +44 (0) 1865 275 413 Fax: +44 (0) 1865 275 410 2 Schlumberger Cambridge Research, High Cross, Madingley Road, Cambridge, CB3 0EL, United Kingdom. 1 This journal is © the Owner Societies 2004 Electronic supplementary information for Physical Chemistry Chemical Physics Supporting Information In the following section we discuss the electrochemical reduction of TCBQ at a GC electrode in different aprotic solvents, namely DMF, DMSO and PrCN, in the presence of 0.1 M TBuAP as supporting electrolyte and compare the results to those obtained using BN as solvent. Comparison of the electrochemical reduction of TCBQ at GC in BN with other aprotic solvents (DMF, DMSO and PrCN) The reduction of TCBQ was also carried out in other aprotic solvents, namely DMF, DMSO and PrCN in the presence of 0.1 M TBuAP as supporting electrolyte. The corresponding voltammograms (0.1 V s-1) for the reduction of 0.5 mM TCBQ at a GC electrode in each different solvent are overlaid in Figure S1. The potential data are summarised and compared to those obtained in BN in Table S1 along with the Gutmann donor number, DN, and the viscosity, , of each solvent. In all the solvents, two peaks were observed corresponding to the reduction of TCBQ to the radical anion, followed by reduction to the dianion. However, variation in the peak potential for the reduction were seen and a comparison of the reduction peak potential observed for the first process reveals that Epr is less negative for DMF and DMFO as compared to BN and PrCN. Solvent effects in the electrochemical reduction of several p-quinones in aprotic solvents have been reported before [1] and can be 2 This journal is © the Owner Societies 2004 Electronic supplementary information for Physical Chemistry Chemical Physics interpreted in terms of increasing stabilisation of the electrode products (radical anion and di-anion) with increasing donor number of the solvent. The relative order of donor number DN is [2,3]: BN < PrCN < DMF ~DMSO Indeed, a shift towards more positive potentials was found going from the higher electron donating solvents DMF and DMSO (DN=26.6, and 29.8, respectively [2]) to BN and PrCN (DN=11.9 and 16.6, respectively [2,3]). The less magnitude of both signals obtained in DMSO, as compared to that obtained in the other aprotic solvents, might be accounted for the lower diffusion coefficient of TCBQ in DMSO [4] as a result of its higher viscosity (see Table S1, [5]). Figure S1: Cyclic voltammograms (0.1 V s-1) recorded at a GC electrode (3 mm diameter) for the reduction of 0.5 mM TCBQ in homogeneous solutions of DMF, DMSO and PrCN all containing 0.1 M TBuAP as supporting electrolyte. 3 This journal is © the Owner Societies 2004 Electronic supplementary information for Physical Chemistry Chemical Physics Table S1: Potentials (vs. Pt) for the reduction of 0.5 mM TCBQ in different aprotic solvents (0.1 M TBuAP) at a GC (3mm diameter) electrode and solvent donor number (DN, [2,3]) and viscosity (, [5]). Solvent DN /cP DMSO 2 Epr1 Epo1 Emid1 Ep1 Epr2 /V /V /V mV /V /V Epo2 Emid2 Ep2 /V /mV 29.8 -0.193 -0.123 -0.158 70 -1.02 -0.93 -0.97 90 DMF 0.796 27 -0.120 -0.023 -0.071 97 -0.99 -0.89 -0.93 100 PrCN 0.515 16.6 -0.214 -0.112 -0.179 71 -1.11 -1.02 -1.06 90 BNa 1.24 11.9 -0.273 -0.173 -0.233 100 -1.07 -0.97 -1.08 101 a Reduction of 1 mM TCBQ References: [1] K. Sasaki, T. Kashimura, M. Ohura, Y. Ohsaki, N. Ohta, J. Electrochem. Soc., 1990, 137, 2437. [2] W. R. Fawcett, Langmuir, 1989, 5, 661. [3] Y. Zhang, Y. Moriguchi, M. Hashimoto, K. Sakata, Anal. Sci., 2001, 17, 675. [4] M. Gomez, I. Gonzalez, F. J. Gonzalez, R. Vargas, J. Garza, Electrochem. Commun., 2003, 5, 12. [5] J. F. Coetzee, Recommended Methods for Purification of Solvents and Tests for Impurities, Pergamon Press, Oxford, 1982, p. 1-59. 4