Spectra of Light
advertisement

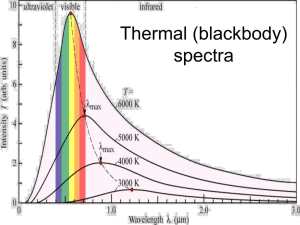
Spectra of Light Spectrum, in optics, the arrangement according to wavelength of visible, ultraviolet, and infrared light. An instrument designed for visual observation of spectra is called a spectroscope; an instrument that a photographs or maps spectrum is a spectrograph. The typical spectroscope is a combination of a microscope and a prism. The prism breaks the light into its spectra components (by differential refraction) which is then magnified with a microscope. Spectra may be classified according to the nature of their origin, i.e., emission or absorption. An emission spectrum consists of all the radiations emitted by atoms or molecules, whereas in an absorption spectrum, portions of a continuous spectrum (light containing all wavelengths) are missing because they have been absorbed by the medium through which the light has passed; the missing wavelengths appear as dark lines or gaps. The spectrum of incandescent solids is said to be continuous because all wavelengths are present. The spectrum of incandescent gases, on the other hand, is called a line or emission spectrum because only a few wavelengths are emitted. These wavelengths appear to be a series of parallel lines because a slit is used as the light-imaging device. Line spectra are characteristic of the elements that emit the radiation. Line spectra are also called atomic spectra because the lines represent wavelengths radiated from atoms when electrons change from one energy level to another. Band spectra is the name given to groups of lines so closely spaced that each group appears to be a band, e.g., nitrogen spectrum. Band spectra, or molecular spectra, are produced by molecules radiating their rotational or vibrational energies, or both simultaneously. What is Spectroscopy? Spectroscopy is a very important tool in astronomy. It is detailed study of the light from an object. Light is energy that moves through space and can be thought of as either waves or particles. The distances between the peaks of the waves of light are called the light's wavelength. Light is made up of many different wavelengths. For example, visible light has wavelengths of about 1/10th of a micrometre - ten thousand wavelengths would be the width of a penny. Spectrometers are instruments that spread light out into its wavelengths creating a spectrum. Within these spectra, astronomers can study emission and absorption lines that are the fingerprints of atoms and molecules. An emission line occurs when an electron drops down to a lower orbit around the nucleus of an atom and looses energy. An absorption line occurs when electrons move to a higher orbit by absorbing energy. Each atom has a unique spacing of orbits and can emit or absorb only certain energies or wavelengths. This is why the location and spacing of spectral lines is unique for each atom. Astronomers can learn a great deal about an object in space by studying it’s spectrum, such as its composition (what its made of), temperature, density, and it's motion (both it's rotation as well as how fast it is moving towards or away from us). There are three types of spectra that an object can emit: continuous, emission and absorption spectra. The examples of these types of spectra shown below are for visible light as it is spread out from purple to red, but the concept is the same for any region of the electromagnetic spectrum. Continuous spectra Continuous spectra (also called a thermal or blackbody spectra) are emitted by any object that radiates heat (has a temperature). The light is spread out into a continuous band with every wavelength having some amount of radiation. For example, when sunlight is passed through a prism, it's light is spread out into it's colours. A continuous visible light spectrum Absorption spectra If you look more closely at the Sun's spectrum, you will notice the presence of dark lines. These lines are caused by the Sun's atmosphere absorbing light at certain wavelengths, causing the intensity of the light at this wavelength to drop and appear dark. The atoms and molecules in a gas will absorb only certain wavelengths of light. The pattern of these lines is unique to each element and tells us what elements make up the atmosphere of the Sun. We usually see absorption spectra from regions in space where a cooler gas lies between us and a hotter source. We usually see absorption spectra from stars, planets with atmospheres, and galaxies. Detailed image of our Sun's visible light spectra The absorption spectra of hydrogen - can you see this pattern in the solar spectrum above this image? (Hint: hydrogen is the most abundant element in the sun - look at the darkest lines). Emission spectra An emission spectra occurs when the atoms and molecules in a hot gas emit extra light at certain wavelengths, causing bright lines to appear in a spectra. As with absorption spectra, the pattern of these lines are unique for each element. We can see emission spectra from comets, nebula and certain types of stars. The emission spectra of hydrogen In practice, astronomers rarely look at spectra the way they are displayed in the above images. Instead they study plots of intensity or signal versus wavelength. These plots show how much light is present or absent at each wavelength. A peak in the plot shows the position of an emission line and dip shows where an absorption line is. The spacing and location of these lines are unique to each atom and molecule. The shape of the continuous spectra (often referred to as the continuum) on a plot is dependent on temperature and motion of the emitting gas. In this simple plot it is shown as a flat line - in reality it is usually a curved line. Also, many of the real data plots you will see have the wavelength or frequency on a logarithmic scale. Examples of spectra Hydrogen: Helium: Carbon: [ Loads more on: http://www.northallertoncoll.org.uk/Physics/Learning%20Resources/Module%201/spectra%2 0web%20links.htm ] Thus, emission spectra are produced by thin gases in which the atoms do not experience many collisions (because of the low density). The emission lines correspond to photons of discrete energies that are emitted when excited atomic states in the gas make transitions back to lower-lying levels. A continuous spectrum results when the gas pressures are higher. Generally, solids, liquids, or dense gases emit light at all wavelengths when heated. An absorption spectrum occurs when light passes through a cold, dilute gas and atoms in the gas absorb at characteristic frequencies; since the re-emitted light is unlikely to be emitted in the same direction as the absorbed photon, this gives rise to dark lines (absence of light) in the spectrum. Hydrogen Emission and Absorption Series The spectrum of hydrogen is particularly important in astronomy because most of the Universe is made of hydrogen. Emission or absorption processes in hydrogen give rise to series, which are sequences of lines corresponding to atomic transitions, each ending or beginning with the same atomic state in hydrogen. Thus, for example, the Balmer Series involves transitions starting (for absorption) or ending (for emission) with the first excited state of hydrogen, while the Lyman Series involves transitions that start or end with the ground state of hydrogen; the adjacent image illustrates the atomic transitions that produce these two series in emission. Because of the details of hydrogen's atomic structure, the Balmer Series is in the visible spectrum and the Lyman Series is in the UV. Web sites: Spectra http://www.colorado.edu/physics/2000/quantumzone/ http://www.ipac.caltech.edu/Outreach/Edu/Spectra/spec.html http://home.achilles.net/~jtalbot/data/nebula/NGC2440.html Bohr Model of the atom http://csep10.phys.utk.edu/astr162/lect/light/bohr.html http://zebu.uoregon.edu/~js/glossary/bohr_atom.html http://www.physicslessons.com/exp28b.htm Quantum theory notes: http://science.howstuffworks.com/atom8.htm Problems of Rutherford theory: http://www.symonds.net/~deep/stuff/qp/chap04.php Bohr applet & notes: http://www.mhhe.com/physsci/astronomy/applets/Bohr/frame.html http://www.upscale.utoronto.ca/GeneralInterest/Harrison/BohrModel/BohrModel.html Loads of revision notes: http://www.jcphysics.com/toolbox_indiv.php?sub_id=23