Notes 10

Notes 10
Thermodynamics is concerned with energy and its transformation. The laws of thermodynamics are general restrictions which nature imposes on all such tranformations.
Can these laws be derived from anything more basic, or are they primitive. If fact, are the words used in thermodynamics primitive?
Energy is such a word.
Kinetic energy of a mass of material is a function of the velocity and has no other reality.
1st Law of Thermodynamics
Formal statement asserting that the energy is conserved.
If a system exchanges energy or rearranges its energy, it likely has a change of state.
An equilibrium state represents a condition of the system. In such a state the system exhibits a set of identifiable reproducible properties that can be detected with instruments.
The importance of the equilibrium is that the system exhibits the properties independent of time and associated with these states are state functions (indep of path) H, U, T, P S, G etc.
A process is then a displacement from equilibrium during which the system's properties change and a new equilibrium state is attained. During the process the system in some cases may interact with its surroundings to exchange heat and work which are considered
"desirable" changes.
Thus for a closed (constant mass) system the 1st Law can be expressed as:
E = Q in
+ W in
= Q in
- W out
E =
E k
+
E p
+
U where
E k
and
E p
are the change in the kinetic and potential energies as a whole.
U is peculiar to thermodynamics in that it represent the individual kinetic and potentials of the molecules, atoms and subatomic particles that constitute the system.
We also define the enthalpy passed on the internal energy, H = U + PV and dH = dU + d(PV). This is defined so that at constant pressure the heat =
H.
2nd Law of Thermodynamics
Nature also places a second restriction on the processes which has to do with the disorder of the system and surroundings. The disorder or randomness is captured in the thermodynamic variable known as the entropy, S.
S tot
=
S sys
+
S surr and in a spontaneous or natural process
S tot
=
S univ
≥ 0
Also remember that the entropy is related to the randomness or the # of ways of arranging a system such that it looks the same after rearrangement (called W)
Where S = klnW k being Boltzmann's constant
It is difficult to use the entropy to know if a process is spontaneous because you must know about not only its change in the system, but also its change in the surroundings.
Remember to solve this, new thermodynamic variables were defined: the Gibbs Free Energy G = H - TS and the Helmholtz Free Energy A = U - TS
The criteria for spontaneity of a process in a system based on these variables were:
G)
T,P sys
≤ 0 or (
A)
T,V sys
≤ 0
One only needs to look at the change in these variables for the system and not the surroundings. Note that they do have some restrictions though.
3rd Law of Thermodynamics
The 3rd Law defines the zero of entropy as the state of a pure perfect crystalline material at 0K.
We as chemists often perform processes in beakers at constant pressure and maybe constant temp. Thus the Gibbs Free Energy is often used as a criterion for spontaneity and it is also then related to the extent of the reaction at equilibrium and the equilibrium constant.
The equilibrium constant is related to
G by the expression:
G = -RTlnK
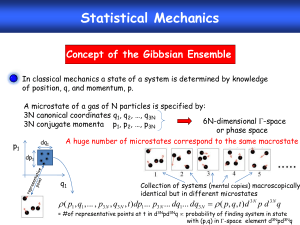
Statistical Thermodynamics
If the bond lengths, bond angles, and bond flexibility of the bonds in a molecule are known, then we'll see that the internal energy of the free energy must be a the value that it is.
Thus one should be ale to bridge the gap between the microcosmic realm of atoms and molecules and the macroscopic realm of classical thermodynamics.
Statistical Thermodynamics (Statistical Mechanics) provides the bridge by forming a thermodynamic system as an assembly of units. More specifically it demonstrates that the thermodynamic parameters of the system are interpretable and calculable from the parameters descriptive of such constituitive units such as atoms and molecules.
The energies open to any of its submicroscopic components, it turns out, are limited to a discontinuous set of alternative values associated with integral values of some Quantum
#s. Thes Q# are related to the electronic, vibrational, rotational, translational, etc. energies, the energies that make up the internal energy in a molecule.
Thus we are going to view a macroscopic thermodynamic system as an assembly of submicroscopic entities with changing quantum states. Seems hopeless, how can we possibly hope to account for even one mole of units. We've seen that a 3 body problem is impossible to solve exactly, so how can one solve 3 x 6 x 10
2 3
body problem?
It is going to turn out that because of the enourmous number of different ways that the internal energy can be arranged, that this problem is tractable when we give it a statistical formulation!
Start off in a simple way. For instance we might be interested in the internal energy of an assembly which is just 3 units which can have vibrational energy. The units are then defied as Distinguishable Operators .
Suppose these oscillators share three quanta of vibrational energy statistically speaking.
How many ways can the distinguishable oscillators share the 3 quanta?
W
I
= 3!/(2! 1!) W
II
= 3!/(1! 1! 1!) W
III
= 3!/3!
Each of the ten ways we'll call a microstate.
This is of general form
For an assembly of (N) localized harmonic oscillators, the # of ways one can distribute among these N oscillators, a particular set of energy parcels which satisfy the predetermined requirements.
N choices of oscillator to assign 1st
N-1 choices of oscillator to assign 2nd etc. = N! distinguishable possibilities
If some of the parcels are the same, then:
W = # of microstates associated with any configuration involving N distinguishable units where n a
are the # of units in one level , n b
equals the number in another quantum level etc.
W = N!/(n a
! n b
! …)
Applicable to any configuration of distinguishable units with any energy spacing between quantum bands
For very very large values of N - need approximation to handle N! lnN! = NlnN - N
What is the # of energy quanta is not equal to the units present?
Look at 4 energy quanta shared among 8 oscillators.
Possible configurations are
W
I
= 8!/(1! 7!) W
II
= 8!/(1! 1! 6!) W
III
= 8!/(2! 6!)
W
IV
= 8!/(4! 4!)
W i
= 400
W
V
= 8!/(1! 2! 5!)
What about 5 quanta in 10 oscillators
Fig shows the configurations corresponding to this.
W
I
= 10!/9!
W i
= 2002 microstates
Note that as the number of places to put the energy increases, the number of microstates increases as well. 1000 oscillators sharing 1000 energy quanta has more thatn 10 6 0 0 different microstates.
For 6 x 10 2 3 oscillators sharing 10 2 3 energy quanta ~ 10 1 0 ^ 2 3 is the number of microstates.
Seems inconceivable that we could figure out all of the possible configurations and determine the # of microstates represented by each.
But it turns out we don't have to do this - NOTE that in going from an 8-4 assembly to a
10-5 assembly there is clearly a predominant configuration arising such that by the 10-5 assembly 42% of the microstates are associated with that assembly.
Statistical analysis shows that the emergence of a predominant configuration is characteristic of an assembly with a large number N and peaking becomes even more protruding. Much like the Poisson Distribution which shows how the # of tosses of a coin affects the probability of ending up with a 50/50 ratio of Heads to Tails
By the time the number of tosses is 1000, most of the 2
1 0 0 0
, approximately 10
3 0 0 microstates are associated with configurations where 45-55% of the tosses are heads.
So expect the same in an atomic system with say 1000 quanta present in 1000 harmonic oscillators.
Important fact is that almost 100% of this area falls under a central, and the sharpness of the peak increases for larger numbers of atoms and molecules.
Thus expect that one can find the configuration with the most # of microstates. Tells us some thing about how the microstates are distributed. How can we find it? If like before this would take forever?
Once found what does it give to us? Tells us that in a macroscopic assembly, an overwhelming proportion of all possible microstates arise from a relatively small # of configurations. Thus the assembly of al will be found always in one macroscopic state that corresponds to the predominant configuration.
One has the criterion for the predominant configuration
W/
= 0 can be used for the predominant configuration
Try it
Consider an isolate Macroscopic assembly of N harmonic oscillators: Identical (but distinguishable because they are at specific locations in space!)
The N harmonic oscillators share a large # of Q energy Quanta - since isolated N and Q are both constant.
What is the predominant configuration?
Consider three successive quantum levels l, m and n where
n
,
m
, and
n
are their energies with n l
, n m
, and n n
oscillators in them
W = N!/ (n a
! … n l
! n m
! n n
! ... n z
! ...)
If make a minimal change in the initial configuration, then N and Q are constant so if n m
=n m
-2 then n l
= n l
+1 and n n
= n n
+ 1
So one can write:
W' = N! / (n a
! … (n l
+1)! (n m
-2)! (n m
+1)! ….n
z
!)
Suppose that the original config was the predominant configuration. In that case dW/d
= 0
So dW = 0 OR W = W'
W = N!/ (n a
! … n l
! n m
! n n
! ... n z
! ...) = W' = N! / (n a
! … (n l
+1)! (n m
-2)! (n m
+1)! ….n
z
!) n l
! n m
! n n
! = (n l
+1)!(n m
-2)!(n n
+1)! n m
! / (n m
-2)! = (n l
+1)!/n l
! (n n
+1)!/n n
! n m
(n m
-1)(n m
-2)! / (n m
-2)! = (n l
+1) n l
!/ n l
! (n n
+1) n n
! /n n
!
n m
(n m
-1) = (n l
+1) (n n
+1) assuming the 1 to be insignificant n m
2
= n l n n n m
/n n
= n l
/n m
these were chosen randomly so if we had chosen m, n, o instead n m
/n n
= n n
/n o
This geometric sequence then describes the predominant configuration for an assembly of harmonic oscillators with uniform spacing.
Does this work in the more general case where the energy spacing is not uniform?
Suppose
l
<
m
<
n and
n
-
m
) / (
m
-
l
) = p/q where p and q are positive integers now if the configuration has aslight change, which holds invariant, the total number of units and amount of energy (ie p+q units) from level m transfer q of them to level n and p of them to level l. this maintains constant total energy since: q
n
-
m
) = p (
m
-
l
) OR q
n
-
m
) + p (
l
-
m
) = 0
If the original configuration was predominant max W then dW/dx = 0 so
N!/ (n a
! … n l
! n m
! n n
! ... n z
! ...) = N! / (n a
! … (n l
+p)! (n m
-(p+q))! (n m
+q)! ….n
z
!) n m
! / (n m
-p-q)! = (n l
+p)!/n l
! (n n
+q)!/n n
! n m
(n m
-1) ... (n m
-p-q+1) (n m
-p-q)! / (n m
-p-q)! = (n l
+p) ... n l
!/ n l
! (n n
+q) ... n n
! /n n
!
n m
(n m
-1) ... (n m
-p-q+1) = (n l
+p) ... (n l
+1) ... (n n
+1) * (n n
+q) ... (n n
+1) since n l n m n n
>> p,q
Then (n l
+ 1) … (n l
+p) ≈ n l
* n l
* n l
.. = n p
Similarly for the others n m
( p + q )
= n m p n m q
= n l p
n n q so (n l
/n m
) p
= (n m
/n n
) q
Note this reduces to what we had before if p = q
So whatever the energy spacing between quantum levels, the eqn. is applicable to any isolated system of identical but distinguishable units. However, this eqn. is more useful if one takes the natural log of both sides. ln(n l
/n m
) p
= ln(n m
/n n
) q p ln(n l
/n m
) = q ln(n m
/n n
) OR p/q ln(n l
/n m
) = ln(n m
/n n
)
From q
n
-
m
) + p (
l
-
m
) = 0 OR p/q =
n
-
m
) / (
m
-
l
)
So
1/(
m
-
l
) ln(n l
/n m
) = 1/
n
-
m
) ln(n m
/n n
)
Remember l, m, and n are any three quantum levels so could be k, l, m, etc.
Thus whatever the focus, the indicated function must be exactly the same. So it must be a constant, call it
.
1/
i
-
n
) ln(n n
/n i
) =
OR ln(n n
/n i
) =
(
i
-
n
) = -
(
n
-
i
)
OR
Suppose that n i
equals n o
, the ground state. Then ln(n n
/n o
) = -
(
n
-
o
) = -
OR n n
/n o
= e
- β Δ ε
This is that Boltzmann Distribution (STILL NEED
For an isolated macroscopic assembly of any species of identical but distinguishable units with any kind of energy spacing between their quantum states, this defines the explicit functional relationship between the energy and the population of each quantum level.
2)
3)
1)
So with the aid of Boltzmann's Law, one finds that:
"Of the immense total number of microstates that can be assumed by a large assembly, and overwhelming proportion arises from one comparatively small set of configurations centered on, an only minutely different one from the predominant configuration such that
W/W max
≈ 10 - 3 4 for 10 2 3 size assembly (1/6 mole)
Physical Significance of the Predominant Configuration
Basic Postulate of Statistical Thermo
Over long periods of time, every possible microstate of an isolated assembly occurs with equal probability. Essentially ratios of the numbers of observed occurrences of any two microstates will approach unity - can't measure these
For 6 x 10
2 3
units - can't make all measurements instantaneously - even supposing that one could make 10
6
microstate observations per second, it takes (10
6
)
1 0 0
for just 1000 harmonic oscillators sharing 1000 energy quanta.
So the answer here is not directly verifiable, but empirical predictions based on it are verifiable.
Clarify what is meant by the BASIC POSTULATE
4)
5)
6)
7)
Thus although every microstate accessible to a given assembly occurs with the equal probability that doesn't mean that all its configs. have an equal probability of occurrence.
If a system is in a config. where W is small, expect a spontaneous change to configs. for which W is greater. This change occurs until at last the assembly attains a PC config.
The system will thereafter be found with a very high probability in a n empirically unchanging equilb. state characteristic of all those macroscopically identical configs typically described by the Boltzmann Law
This is readily interpretable as progressing forward to a maximum W, the equilibration may also be interpreted as a progression toward configs of maximum disorder
(ENTROPY)
So what may at first appear to be a purposeful drive toward states of maximal disorder, can now be seen to arise simply from the operation of blind chance in an assembly wher all microstates remain equally probable, but where an overwhelming proportion of all microstates is ascribed with the maximally disordered predominant configurations.
OVERALL CONCLUSION
Ina an isolated macroscopic assembly, the equilibrium config. is typically that described by the Boltzman Distibution Law
What is
? What is its physical significance?
Does it have any connection with the internal energy?
The energy of the assembly does give information about the value of W. If the energy context is small, then, the units will have most likely no energy quanta. In the limit of no energy - no internal energy then
W = N!/n o
! and n o
= N so W=1.
If there is more energy, then some units have 1,2,3, or mare energy quanta in them, so have n
1 n
2 n
3
W = N!/(n o
!n
1
!n
2
! … )
Here W will be increased, and thus W is related to the internal energy of the system.
W must be related to both S and U.
How?
Consider a macroscopic body of loosely coupled distinguishable units. Put in some heat so energy is increased. this causes a shift from the predominant configuration to a new configuration infinitesimally different from the original one. initially W = N! /
n n
! lnW = ln N! - ln
n n
!
= ln N! -
ln n n
! dlnW = -
dln n n
! Since N! is constant
Use Stirlings approximation
dlnW ≈ - n
ln n n
dn n
For predominant configuration (PC)
Boltzmann's law says that n n
/n o
= e
- β Δ ε ln n n
= ln n o
-
So for the PC dlnW =
Since the total # of untis is a constant
n
dn n
= dN = 0
Also n o
o
+ n
1
1 +
… = n
(n n
n
) = E for small energy transfer as heat and no change in energy levels
n
n dn n
= dE so dlnW =
dE
Assumptions
1) Distinguishable Units
2) No shift in energies of quantum states
3) No chemical or physical change altering the # of units present
4)
constant
We find
= kT k = Botzmann's constant by considering the approach to equilibrium d(W x
W y
) ≥ 0
'
Remembering Thermodynamics, the only time heat flows from y to x without extra intervention is if T x
≤ T y
Close to saying something about the entropy here. Since
S tot
≥ 0 for an isolated system our condition of spontaneity in terms of the # of microstates made from the combination of x and y was that dlnW x
+ dlnW y
≥ 0
Similar to
S
These have striking similarities as Boltzmann thought.
S = klnW
In thermodynamics, differences in state functions are normally of interest
G,
H,
S,
What does the statistical entropy give as T -> 0
Now what about relating this to other thermodynamic variables besides S
Need A Partition Function
Boltzmann law describes the predominant (equilibrium) configuration for an isolated macroscopic assembly of distinguishable untis n i
= n o
e
- β Δ ε
N = n o
+ n
1
+ n
2
+ …
Find that N = n o
(e - β ε o + e - β ε 1 + … ) so n o
= N /
q
e
- β ε q
Substituting in to Boltzmann n i
= n o
e
The
q
e
- β ε q is called the partition function. Tells how the units will partition themselves over the quantum states that are possible. The symbol for the partition function is small z
Thus the partition function "z" expresses the sum of a potentially infinite series that converges more rapidly, the lower the temp. and larger the energy spacing!
Formulation of the Partition function
Consider if applicable to real system. Have assumed that we could assign a unique
"private" energy to each unit of an assembly. Clearly this fails in a highly interactive system.
Some interaction must take place but tot get to equil., so talk about weak coupling.
Applicable to real gas at moderate temps.
A polyatomic gas has many ways to store energy internally. Store in translational, rotational, vibrational, motions or in the form of electronic excitations.
Use Born Oppenheimer like approach. z tot
=
q
e
- β ε q =
q
e
- β ( ε t r a n s
+ ε r o t
+ ε v i b
+ ε e l e c
) z comb
= z trans
* z rot
* z vib
* z elec
Degeneracy z trans
is indistinguishable so it needs to be modified z trans
= 1/N! z
N
Thermodynamics and the Partition Function
U = n o
ε + n
1
ε
1
+ n
2
ε
2
+ …
=
o
N/z e
- β ε o
+
Find that U = - N dlnz/dβ
More generally U = -dlnZ/dβ for distinguishable units z
N
= Z for indistinguishable units z
N
/N! = Z
Note the assumption that -d/d
e
- β ε i
=
i
e
- β ε i
For translational motion this is sketchy since
i
depends on the temp! So need to restrict this to constant volume.
U = (-dlnZ/dβ)
V
= kT
2
[dlnZ/dT]
V
Also
S = -klnW and ln W = lnN! - ln
n q
! = = lnN! -
q lnn q
!
Using Stirlings
Using Boltzmann
So S = klnZ + U/T
S = d/dT [kTlnZ]
V
Can also look at other Thermodynamic Functions
A = U - TS
A = -kTlnZ tot
H = U + PV
G = H - TS
Here it should be stated that many of the thermodynamic parameters are expressible in terms of the partition function. One form of the partition function that we looked at was:
Z tot
= [1/N! (z trans
)
N
(z rot
)
N
(z vib
)
N
(z elec
)
N
]
For an assembly of any species of weakly coupled units with any degrees of freedom!
The forms of z trans
z rot
z vib
z elec
simply follow from the general expression for z z =
i
g i
e
- ε i
/ ( k T )
To get the various
I
, use the quantum mechanical expressions for each of these types of energy.
Examples