Worksheet - Radioactivity 1 Solutions
advertisement
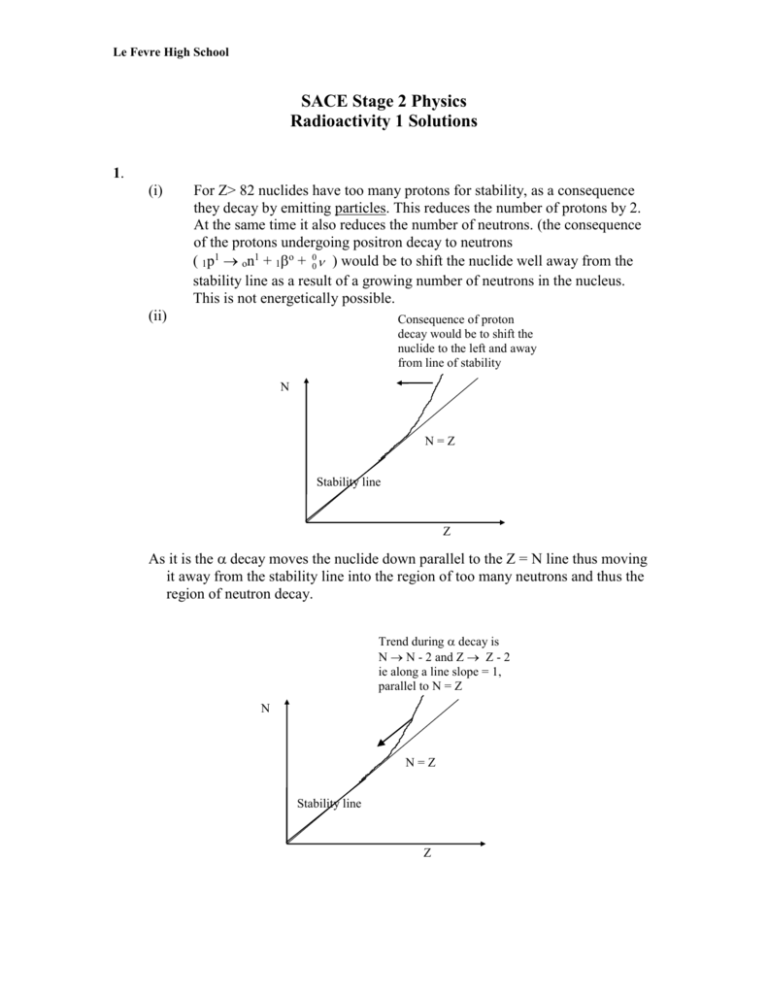
Le Fevre High School SACE Stage 2 Physics Radioactivity 1 Solutions 1. (i) For Z> 82 nuclides have too many protons for stability, as a consequence they decay by emitting particles. This reduces the number of protons by 2. At the same time it also reduces the number of neutrons. (the consequence of the protons undergoing positron decay to neutrons ( 1p1 on1 + 1o + 00 ) would be to shift the nuclide well away from the stability line as a result of a growing number of neutrons in the nucleus. This is not energetically possible. (ii) Consequence of proton decay would be to shift the nuclide to the left and away from line of stability N N=Z Stability line Z As it is the decay moves the nuclide down parallel to the Z = N line thus moving it away from the stability line into the region of too many neutrons and thus the region of neutron decay. Trend during decay is N N - 2 and Z Z - 2 ie along a line slope = 1, parallel to N = Z N N=Z Stability line Z Le Fevre High School (ii) - decay occurs when a nuclide has too many neutrons for stability. This occurs in the region above the stability line in an N versus Z graph. i.e. when N > Z. In this case the neutron decays to a proton so Z goes to Z + 1 1 1 o 0 on 1p + -1e + 0 (proton stays in nucleus) eg. 82Pb210 83Bi210 + -1eo + 00 Trend during decay is N N and Z Z + 1 ie along a line parallel to Z axis N N=Z Stability line Z (iii) + decay occurs when a nuclide has too many protons for stability. This occurs in the region below the stability line when N is plotted against Z. i.e. when N<Z. In this case the proton decays to a neutron decreasing Z by 1 1 1 o 1p on + 1e + (neutron stays in nucleus) eg. 7N13 6C12 + 1eo + Trend unstable nuclide, too many protons, N unchanged, Z Z - 1 N N=Z Stability line Z 2. It often happens that the products of , - or + decay are left in excited states. If the daughter nucleus is left in an excited state it will immediately undergo a transition to a lower energy. This behaviour is similar to that of an atomic system except that the energies involved are much higher. When the nucleus falls from a state of higher to a state of lower energy ray photon rather than a Le Fevre High School visible photon is emitted. It is in this way that the rays emitted by radioactive substances arise. This also explains why the rays have discrete energies. The following diagram illustrates the decay of 83 Bi212 (Bismuth) to thallium 81Tl208. Six different energy particles are emitted accompanied by five different energy ray photons. (no photon is emitted for as it leaves thallium in the ground state). The energies shown are measured relative to the ground state of thallium 6 5 4 3 2 1 0.617 MeV 0.492 MeV 0.472 MeV 0.328 MeV ray emitted 0.328 MeV 0.040 MeV E1 = 6.086 MeV 0 MeV The reaction is 83Bi212 81Tl208 + 2He4 (If you are astute you may note that some energy is missing 6.086 - 5.481 = .605 not .617 MeV. This is because the thallium nucleus also takes off some energy.) 3. Naturally occurring radioactivity is essentially a result of heavy nuclei which have Z > 82. e.g. U238 and U235. These decay by decay into daughter nuclei. 92 92 Some of these heavy nuclei have a long half-life and hence are still present on the Earth's surface. The daughter nuclei are unstable and also decay by either decay or - decay because they have an excess of neutrons. This process continues until a stable nuclide results. Consequently the naturally occurring radiations are , - and . (the accompany both the and - modes of decay.) 4. (a) (i) 6C12 7N14 + -1eo + o o (ii) 88Ra226 86Rn222 + 2He4 ( Rn is Radon ) 13 (iii) 7N 6C13 + 1eo + oo (iv)13Al29 14Si29 + - + o o (v) 13Al24 12Mg24 + + + oo Le Fevre High School (b) aluminium has one stable isotope, (13Al27).13Al29 has an excess of neutrons and so decays by neutron decay ( - decay) whereas 13Al24 has too many protons for the 11 neutrons in it. The 11 neutrons are unable to compensate for the Coulomb repulsive force on the charged protons. Consequently the protons decay to neutrons ( + decay). (c) radiation. Some nuclei will be left in an excited state. The excess energy will be lost as radiation when the nuclei drop to their more stable ground state. 5. (a) on1 1p1 + (b) 1p1 on 1 o -1e o 1e + o o + ono 6. 40 20Ca40 + -1eo + o o 19K 192 76Os188 + 2He4 78Pt 210 81Tl206 2He4 83Bi 210 83Bi 24 13Al o 210 o 84Po -1e + o 24 + + + ono 12Mg 7 (a) (i) (ii) N 13 6 C 13 + +1 e 0 + 0 0 210 83 Bi 210 + -1 e 0 + 0 0 82 Pb 7 (b) In any decay reaction the mass of the products is less than the mass of the parent nucleus. Some of the parent mass has been converted into energy. The mass defect and hence the total released energy is constant and unique for a given reaction. E max is a measure of this energy and so is also constant (note that the daughter nucleus carries off an insignificant proportion of the energy released as it is so massive compared to an electron.) (c) The continuous spectrum of particle energies indicates that most the particles are emitted with less energy than Emax. Experiments show that they have not lost the energy in collisions with other atoms on the way out. This means that if mass-energy is to be conserved the missing energy had to be carried off by another particle which does not show up readily in experiments. To be so "invisible" this particle must have zero rest mass no charge very little interaction with matter The particle is called the neutrino. Le Fevre High School 8. From conservation of momentum Pbefore = P after 0 = P + PD 0 = m v + mD vD v mD vD m v mD In size, then, = vD m Hence (0.5)m v 2 E = ED (0.5)mD v D 2 m v = mD v D m = mD mD m 2 2 E mD = ED m (b) From conservation of energy E + ED = Q but ED = mD = Q m E + E mD = Q E 1 m mD m E = Q mD (c) mD Q E = mD m mD Q E = mD m 208 = Q 208 4 208 = Q 212 = 98.1 % Q mD E m from (a) Le Fevre High School 9. Measurements of the energy spectrum of and rays show that they have "line spectra", i.e. a few discrete values of and ray energies are associated with each decaying nuclide. Analysis of the energies involved (taking into account the fact that the particle does not carry all of the energy released in a reaction) suggests that the daughter nucleus may be formed in a stable or one or more excited states. If left in an excited state excess energy is released by emission of ray photons as the nucleons in the daughter nuclei rearrange themselves into the most stable ground state. The following energy level diagram is based on measurement of the radiations emitted in the decay of Bi212 to Thallium208 Bi ground state 6.20 E = 5.71 MeV 0.49 0.47 E = 6.20 MeV Tl excited states Tl ground state 0.33 MeV above ground state 0.04 0 An particle with energy 98 % of each of these values is released. rays corresponding to each of these transitions are observed This strong inter-relation of the energy of the emitted and ray particles provides the evidence for the existence of energy levels.