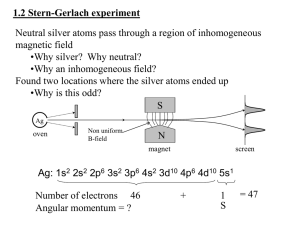
1-18
advertisement

ANISOTROPIC EXCHANGE INTERACTION IN A SPINCANTED SINGLE CHAIN MAGNET BASED ON Co(II) IONS Andrei Palii, Oleg Reu, Sergei Ostrovsky, Sophia Klokshner Institute of Applied Physics, Academy of Sciences of Moldova, Kishinev, Moldova Kim Dunbar Department of Chemistry, Texas A&M University, College Station, TX, USA Boris Tsukerblat Chemistry Department, Ben-Gurion University of the Negev, Beer-Sheva, Israel We report a model of a single-chain magnet Co(H2L)(H2O) (L = 4-Me-C6H4CH2N(CPO3H2)2) based on Co(II) ions with unquenched orbital angular momenta. The model includes a tetragonal crystal field, spin-orbit interaction acting within each Co(II) ion, an antiferromagnetic Heisenberg exchange between the Co(II) ions and the titling of the tetragonal axes of the neighboring Co units in the zigzag structure. The titling of the anisotropy axes gives rise to a spin canting and consequently to a nonvanishing magnetization of the chain. The effective pseudo-spin-1/2 Hamiltonian for the interaction between the Co ions in their ground Kramers doublet states proved to be of the Ising type. The model provides a perfect fit to the experimental data for static and dynamic magnetic properties of the chain. 1. INTRODUCTION One-dimensional systems, exhibiting magnetic bistability, commonly referred to as single chain magnets (SCMs) are of high interest due to their unusual physical properties and potential importance for high-density data storage and quantum computing [1,2]. During last years this branch of molecular molecular magnetism dealing with the 1D magnets is being intensively developed [2-13]. The background for the description of SCM is provided by the Glauber’s stochastic approach [14]. Glauber predicted the presence of a slow relaxation of magnetization in a chain composed of ferromagnetically coupled spins that can be described by the Ising Hamiltonian: H ex 2 J Z i Z j , (1) i j where Z is the Z -component of the pseudo-spin operator. In Glauber’s theory of the thermal variation of the relaxation time is described by the Arrhenius low b k BT ( T ) 0 exp (2) in which the barrier b to reverse the magnetization direction represents the energy loss in one spin flip-flop process, that is 1-18 b 2J . (3) An Ising spin chain can behave as SCM if the constituent magnetic moments are not canceled. In majority of known SCMs this condition is satisfied either due to ferromagnetic interaction between identical spins or due to alternation of different antiferromagnetically coupled canted spins. Recently an unusual SCM composed of antiferromagnetically coupled metal ions with the canted spin structure has been reported [13]. This system represents the cobalt(II) diphosphonate Co(H2L)(H2O) compound (L = 4-Me-C6H4-CH2N(CPO3H2)2) in which the Co(II) ions are linked through a bridging phosphonate oxygen atom to create a 1D chain of corner-sharing octahedra which propagate in a zigzag fashion (Fig.1). This compound is the first SCM based on antiferromagnetically coupled homospin centers in which nonvanishing magnetization appears due to non-collinear spin structure (spin canting). Fig. 1. A zig-zag chain structure of the Co(H2L)(H2O) compound. The 4-Me-C6H4CH2-groups of the diphosphonate ligands have been omitted for the sake of clarity. The Co octahedral and CPO3 tetrahedral units are shaded in green and pink, respectively. The aim of this article is to give a quantum-mechanical description of the magnetic anisotropy and spin-canting phenomena in this system [15]. We elaborate a relatively simple model that incorporates the main sources of the magnetic anisotropy, namely strong axial crystal fields acting on the Co(II) ions, spin-orbital interaction and the topology of the chain. All these factors give rise to a canted spin structure and subsequently to an uncompensated magnetic moment. Finally, we demonstrate that the model perfectly agrees with the experimental data on the static and dynamic susceptibility. 2. THE MODEL The two lowest terms of a free Co(II) ion arising from the 3 d 7 configuration are 4 F (ground) and 4 P separated from 4 F by the gap 15B (Racah parameter) that is typically about 15,000 cm-1 . Octahedral ligand field splits 4 F atomic level into two orbital triplets terms 4T1 , 4T2 and the orbital singlet 4 A2 in such a way that the orbital triplet 4T1 becomes the ground state meanwhile the excited 4 P state results in the T1 . In addition two 4T1 terms (momenta l 1 ) are mixed by the cubic ligand field but 4 the ground state that is mainly of 4 F character and contains also an admixture of 4 P . Since the spin canting is closely related to the magnetic anisotropy the model should incorporate both spin-orbital interaction and local non-cubic crystal field. Let 1-19 us first consider the Co(H2L)(H2O) unit (Fig. 2). The Co-ligand distances in the first coordination sphere of the Co(H2L)(H2O) unit (Fig. 2) are collected in the Table [13]. Fig. 2. ORTEP representation of the Co(H2L)(H2O) unit. Table . Bond lengths [Å] and angles [deg] for the nearest Co surrounding in Co(H2L)(H2O) (data from ref. [13]). ____________________________________________________________________ Co(1)-O(22)#1 2.035(5) Co(1)-O(1W) 2.098(5) Co(1)-O(13) 2.134(5) Co(1)-O(23)#2 2.139(5) Co(1)-O(23) 2.176(5) Co(1)-N(1) 2.282(6) The nearest octahedral surrounding of the Co(II) ions is strongly distorted so that the actual symmetry is very low. But considering the fragment of the structure involving Co(II) and the nearest surrounding one can see that a simplifying assumption about tetragonal character of distortion can be made with a reasonable accuracy. The tetragonal axis is expected to coincide with the N-Co-O diagonal in the distorted hetero-ligand coordination sphere that can be approximately described by C4v point symmetry group. Let us assign the indices A and B to two octahedrally coordinated Co(II) ions which occupy non-equivalent crystallographic positions in a 1D chain. Let us introduce two local frames of reference (Fig. 3) related to ions A and B in the chain. We assume that the tetragonal local Z A and Z B axes subtend an angle , the Y A and YB axes are chosen to be parallel to each other and perpendicular to Z A Z B -plane, while the axes X A and X B lie in the Z A Z B -plane. The local axes for B center can be obtained from those related to A center axes by the turn through angle around Y A or YB axis. A tetragonal component of the ligand field splits the ground 4 T1 term of the Co(II) ion in C4v symmetry into the orbital singlet 4 B2 and the orbital doublet 4 E . The axial ligand field and the spin orbit interaction is described by the following single-ion Hamiltonian [16, 17] : H Co p l Z2 p 2 3 3 2 l s , p A, B (4) where l Z p are the projections of the orbital angular momentum operators onto the local κ takes into account both the covalence effects and the mixing of 4T1 4 F and 4T1 4 P terms by the cubic crystal field. The Z -axes, and the orbital reduction factor 1-20 factor 3 2 in eq. (4) is conventionally introduced in the matrix of the angular momentum operator due to the fact that the matrix of l̂ within 4 T1 4 F coincides with the matrix of 3 2 l̂ defined in the atomic p -basis. manifold XB A XA B ZA 2 2 ZB X Z Fig. 3. Local and molecular co-ordinates. The tetragonal ligand field defined by the first term of eq. (4) stabilizes the 4 B2 term (state with ml 0 ) in the case of positive tetragonal field 0 and the 4 E term ml 1 when 0 . The spin- orbit coupling produces further splitting of these levels into the Kramers doublets. In general the axial crystal field and spin-orbital interaction are to be taken account simultaneously. In order to elaborate a model that would incorporate these main physical factors and at the same time would be relatively simple we note that the geometry of a strongly distorted hetero-ligand surrounding of Co(II) ion in Co(H2L)(H2O) (Table) seems to provide an argument in favor of a simplified model based on the assumption of a strong axial field. In this context we will assume that the fields exceeds considerably the spin-orbital interaction, . We have to discuss which sign of is relevant . In the strong positive axial field limit the ground term 4 B2 is orbitally non-degenerate (conventionally, spin system) so that the firstorder orbital angular momentum is quenched. However, the observed room temperature T value can be considered as an indication of the presence of an unquenched orbital momentum of the Co(II) ion in the Co(H2L)(H2O) compound. Actually, the observed value of T at 300K is 3.2 emu·mol-1 K is higher than the expected value CoT = 1.875 emu·mol-1 K for a spin system. The case of 0 seems to be incompatible with the observed SCM properties of the compound. In fact, such type of behavior can be observed in the chains composed of the magnetically coupled Ising spins. This means that the case of positive axial field can be excluded from the further consideration. Another argument in favor of relevance of the case of 0 is discussed below (see Section 4). For this reason we focus on the case of 0 when the ligand field stabilizes the orbital doublet 4 E . Assuming that the axial field considerable exceeds the spin orbital coupling (axial limit) and neglecting the spin orbital mixing of the 4 E and 4 B2 terms we arrive at the scheme of the energy levels shown in Fig. 3. The spin orbit interaction takes an axial form with the only non-vanishing Z- component p 4 H SO E 3 2 l Z p s Z p 1-21 within the ground 4 E term and leads to the splitting of this term into four equidistant Kramers doublets ml 1, ms 3 2 , ml 1, ms 1 2 , ml 1, ms 1 2 , ml 1, ms 3 2 as shown in Fig. with the with ml 1, ms 3 2 state being the ground one. This is an immense simplification but we will show that the experimental data can be perfectly explained within a model so far discussed. Along with the local frames we will also use the molecular coordinate frame chosen in such a way that the molecular Z axis is directed along the bisector of the angle formed by the local Z A and Z B axes while the Y axis of the molecular system coincides with the local Y A and YB axes (Fig. 2). The full Hamiltonian of the Co(II) pair includes the intracenter interactions described by eq. (4) and the exchange interaction between Co(II) ions. In general, the interaction between orbitally degenerate ions is described by the so-called orbitally dependent exchange Hamiltonian Following the the approximation proposed by Lines [16,17] and discussed in the subsequent papers [18] dealing with the orbitally dependent superexchange in Co(II) we assume here that exchange interaction is represented by the isotropic Heisenberg-Dirac- Van Vleck (HDVV) Hamiltonian H ex 2 J s A s B , (5) where J is the exchange parameter, the single ion spin operators s A and sB ( s A s B 3 2 are the spins of a Co(II) ion) and their projections s A and s B X ,Y , Z refer to the molecular frame. It is convenient to pass from the operators s A , s B to the operators s A , s B defined in the local frames [18]. s X A cos 2 0 sin 2 s X A 0 1 0 sY A sY A , s A sin 2 0 cos 2 s Z ZA (6) s X B cos 2 0 sin 2 s X B 0 1 0 sY B sYB s B sin 2 0 cos 2 s Z ZB . Using these matrices one can present the exchange Hamiltonian as follows: H ex 2 J sYA sYB cos s X A s X B s Z A s Z B sin s X A s Z B s Z A s X B .(7) The Hamiltonian in Eq. (7) acts within the full basis set formed by the ground state basis of the two Co(II) ions, i.e. direct product of two 4T1 bases (144x144 matrix). The energy gap between the ground Kramers doublet with ml 1, ms 3 2 and the first excited one ( ml 1,mS 1 2 ) is assumed to exceed the exchange splitting so at low temperatures we can restrict ourselves by considering only the ground 1-22 4 A2 ml 0 T1 ml 0, 1 |Δ| 4 ml 1, ms 3 2 3 | | 2 4 E ml 1 3 | | 2 3 | | 2 Tetragonal crystal field ml 1, ms 1 2 ml 1, ms 1 2 ml 1, ms 3 2 Tetragonal crystal field + spin orbit coupling Fig. 3. Splitting of the ground cubic 4T1(3d7) term of the Co(II) ion by a tetragonal crystal field and spin-orbit coupling in the limit of strong negative tetragonal field (spin-orbital mixing of 4B2 and 4E terms is neglected). Kramers doublet for each Co ion All matrix elements of the operators s X A , sYA , s X B and sYB are vanishing within the basis set of the ground Kramers doublet. ml 1, ms 3 2 s X A ml 1, ms 3 2 0, ml 1, ms 3 2 s X A ml 1, ms 3 2 0, etc (8) and hence the Hamiltonian, eq. (7) dealing with the “true” Co(II) spins (s=3/2) is reduced to the Ising form for the pseudo-spins s ef 1 2 : H ex 2 J eff Z A Z B , (9) Although in the adopted model the mixing of the Kramers doublets by the exchange interaction is also assumed to be small this mixing is taken into account as the correction in the evaluation of the effective exchange parameter J eff : J cos J eff 9 J cos 1 . 3 | | (10) The term proportional to J 2 in eq. (10) represents the second order correction arising from the mixing of the ground and excited manifolds of the cobalt pairs by the exchange interaction. One can see that the new exchange parameter reflects the geometry of the zig-zag chain through the angle meanwhile in the adopted approximation it is independent of axial crystal field. It is worthwhile to note that in the framework of the assumption so far adopted the effective exchange vanishes if the local axes are orthogonal 2 and reach the maximum value 9 J in the linear geometry when the local axes coincide. This provides a possible receipt for a chemical control of the magnetic properties of these kind of 1D compounds. While 1-23 deriving eq. (9) we passed from the true spin-3/2 operators s Z A , s Z B to the pseudospin-1/2 operators Z A , Z B . The pseudo-spin-1/2 basis is chosen in such a way that the component of the ground Kramers doublet level with ml 1 , ms 3 2 ms 3 2 corresponds to the projection 1 2 1 2 of the pseudospin-1/2. Providing such choice the pseudo-spin-1/2 Hamiltonian in the presence of the external magnetic field is found to be [19]: ml 1 , eff p g|| Z p H Z p H 2X p HY2p , H Co p A, B , (11) where H Z p etc are the components of the magnetic field in the local frames. The principal values of the effective g –tensor for the Co(II) ion in their local surroundings are found as: g || 3 g e , g 0 , (12) where the g|| is related to the local Z-axes (along tetragonal axes) and g to the local XY planes. One can see that the system is highly anisotropic and in particular first order Zeeman splitting disappears in the perpendicular field. The values g e2 2 2 | | || 0, (13) are the principal components of the tensor of the Van-Vleck temperature independent paramagnetism (TIP). Using these results we can write down the following total Hamiltonian for a chain including exchange and Zeeman terms: H 2 J eff i i i i 1 g i H ZA ZB ZB ZA || ZA ZA Z B i H Z B , (14) i where index i numbers the AB pairs and the TIP contribution will be added later on. In eq. (14) both the pseudo-spin operators and the components of the magnetic field are defined in the local frames. To get insight on the spin structure of the system one can pass to with the aid of the following relations Z i sin 2 XA i cos 2 ZA i , Z i sin 2 XB i cos 2 ZB i , (15) A where XA i , H 2 J eff ZA i , XB i , B ZB i . Then the exchange Hamiltonian takes on the form cos 2 i i i i 1 2 i A Z B Z B Z A Z sin 2 XA i XB i XB i XA i 1 2 12 sin XA i ZB i ]Y [ ZB i XA i 1]Y g A ZZ B A B ZA g ZZ ZB g XZ XA g XZ XB H Z A B A B g XX XA g XX XB g ZX ZA g ZX ZB H X , 1-24 (16) where A A i , X , Z , and XA i ZB i is the vector product. Finally the i components of the g-factors are given by: A B g ZZ g ZZ g|| cos 2 2 , A B g XX g XX g|| sin 2 2 , A A B B g XZ g ZX g XZ g ZX (17) 1 2 g|| sin One can see that after the projection of the isotropic exchange interactions, Eq. (16), between “true” Co(II) spins onto the restricted space of Kramers doublets one arrives at the strongly anisotropic pseudo-spin-1/2 interaction that gives rise to a noncollinear spin structure. 3. THE BARRIER AND MAGNETIC BEHAVIOR OF THE Co-CHAIN Similarity between the Hamiltonian, eq. (14), and true Ising Hamiltonian provides a simple way of finding the relation between the barrier height and the effective exchange parameter. Let us consider, for example, one spin flip-flop process schematized in Fig. 4 (the spin of the center B in an AB pair is overturned) in the absence of the external magnetic field. The energy loss in such process can be obtained as b E ... B 1 12 E ... B 1 12 2 | J eff | , (18) where the only spin projection B 1 is changed while the remaining ones keep their values (all spin-projections are defined in the local frames). We thus obtain the same relation as that derived from the true Ising Hamiltonian. X B A B XA ZB XB A ZA Z Fig. 4. Non-collinear spin structure of the chain and illustration for a spin flip-flop. In order to calculate the magnetic susceptibility it is convenient to present the total Hamiltonian of the chain in the presence of the external magnetic field applied along the molecular Z axis in the following form: 1-25 H H || Z 2 J eff i i i i 1 ZA ZB ZB i ZA (19) g|| cos 2 H Z Z A i Z B i , i where the spin operators are defined in the local frames, meanwhile the magnetic field is defined in the molecular one. The Hamiltonian in eq. (19) is of the Ising form and therefore one can use the analytical expression for the free energy of a chain [21]. Using the expression from ref. [19] one obtains: F H || Z N k BT ln exp exp cosh J eff 2 k BT sinh 2 g|| cos 2 H Z 2 k BT J eff k BT g|| cos 2 H Z 2 k BT exp J eff k BT (20) When the magnetic field is applied along the molecular X axis the Hamiltonian of the system can be presented as follows: H H || X 2 J eff i i i i 1 ZA ZB ZB i ZA (21) g|| sin 2 H X Z A i Z B i , i For this direction of the magnetic field the free energy is given by the expression J F H || X N k B T ln exp 2 keffBT cosh g || sin 2 H X 2 k BT J J g sin 2 H exp k effT sinh 2 || 2k T X exp k effT B B B . (22) Using these expression one can calculate the principal values of the magnetic susceptibility tensor [18] and also the TIP contribution in a standard way. 4. RESULTS AND DISCUSSION The temperature dependence of the relaxation time for the Co(H2L)(H2O) compound was obtained from the frequency dependence of the in-phase and outof-phase ac susceptibility [13]. The experimental data for obtained in this way were fit to the Arrhenius expression. The experimental and calculated ln 1 vs 1 T dependences are shown in Fig. 5. The best fit parameters were found to be b 18.6 cm 1 , 0 3.4 10 9 s for the in-phase signal of frequency-scan and b 20.2 cm 1 , 0 8.4 10 10 s for the out-of-phase signal, respectively. Then, considering a simple average 1 b b b 2 19.4 cm as a reasonable value of the barrier height one can find from eq. (18) that J eff 9.7 cm 1 . Magnetic susceptibility measurements performed on a polycrystalline sample of the compound at the field H 0.1 T over the temperature range 2–50K revealed the behavior shown in Fig. 6, which is quite similar to that observed in the ferrimagnetic spin chains. As the temperature is lowered, the T value decreases and 1-26 reaches a minimum of 0.6 emu mol 1K at 7 K. Below 7K, T increases abruptly to reach a maximum at ~2.5 K ( Tmax 2.5 emu mol 1K ) and finally decreases again at lower temperatures. The observed increase of T below 7K can be attributed to the finding that antiferromagnetic coupling does not lead to the exact cancellation of the magnetic moments due to spin canting. In the calculation of T we use the values 180 cm 1 , 0.8 that are J eff 9.7 cm 1 obtained from the typical for the high-spin Co(II) ion and Arrhenius plot. Since the actual symmetry of the Co(II) sites is low the angle between the easy anisotropy axes of A and B ions are allowed to be changed in course of the fitting to the experimental T vs. T curve. The best fit is achieved for the angle 15o . Figure 6 shows a perfect agreement between the observed and calculated T vs. T curves thus indicating that the presented theory adequately describes simultaneously both dynamic and static magnetic properties of the compound. Figure 7 displays the ZZT vs T and XX T vs T curves calculated with 180 cm 1 , 0.8 , J eff 9.7 cm 1 and 15o . These dependences demonstrate that the magnetic moments along the Z axis are fully cancelled meanwhile uncompensated magnetic moment appears along the X axis resulting in a distinct maximum on the XX T vs T curve. Hereunder we will provide an additional justification of this assumption and demonstrate that the appearance of the uncompensated magnetic moment along the X axis can not be explained assuming that is positive. Figure 8 shows the easy planes of magnetization for the ions A and B, which coincide with the local X i Yi planes. In Fig. 8 these planes are assigned as planes and for the centers A and B , respectively. In the absence of the exchange interaction between Co ions all orientations of spins τ A and τ B within the corresponding easy planes are energetically equivalent. 9 Ln(1/) 8 7 6 5 4 0,44 0,46 0,48 0,50 0,52 0,54 0,56 0,58 -1 1/T (K ) Fig. 5. Temperature dependence of the relaxation time. The triangles and diamonds represent the relaxation time obtained from frequency dependence of and , respectively. The solid line corresponds to the best fit of the data to eq. (25). Modified from ref. [13]. 1-27 3,0 emu K mol -1 2,5 2,0 1,5 1,0 0,5 0,0 0 10 20 30 40 50 T, K Fig. 6. Temperature dependences of T for Co(H2L)(H2O) compound: triangles experimental data, solid line – theoretical curve calculated with 180 cm 1 , 0.8 , J eff 9.7 cm 1 and 15o . 9 8 emu K mol -1 7 6 ZZT 5 4 3 2 1 XXT 0 0 10 20 30 40 50 T, K Fig. 7. Temperature dependences of the nonzero diagonal components of the T tensor calculated with 180 cm 1 , 0.8 , J eff 9.7 cm 1 and 15o . In the presence of the antiferromagnetic exchange between Co ions the exchange interaction tends to orient the interacting spins antiparallel. There is an unique possibility to minimize both the single ion and the exchange energy, namely to 1-28 align the spins τ A and τ B antiparallel along the crossing line of the planes and (molecular Y axis). As a consequence, the magnetic moments of ions A and B cancel each other and the total magnetic moment vanishes. On the contrary in the case of negative the spins τ A and τ B at low temperatures tend to align along the local easy axes of magnetization provided that the local anisotropy is strong enough to be suppressed by the exchange interaction. These easy axes for the neighboring ions are nonparallel and the resulting nonzero magnetic moment appears along the molecular X axis due to the spin canting effect (Fig.4). Therefore we arrive at the conclusion that our initial assumption about the negative sign of the axial field parameter is the way to explain the magnetic behavior of the Co(H2L)(H2O) compound. Y A yA B yB zA xA A 2 xB B X zB Z Fig. 8. Illustration for the cancellation of magnetic moments of ions A and B in the case of positive axial crystal field CONCLUSION In this article we have presented a quantum-mechanical approach to the description of the SCM behavior and spin-canting phenomena in a zig-zag 1D compound Co(H2L)(H2O) (L = 4-Me-C6H4-CH2N(CPO3H2)2) based on the antiferromagnetically coupled Co(II) ions with unquenched orbital angular momenta. We elaborate a relatively simple model that accounts for the main sources of the magnetic anisotropy, namely strong axial crystal fields acting on the Co(II) ions, spinorbital interaction and the topology of the chain. The pseudo-spin-1/2 Hamiltonian is deduced that contains ferro-antiferromagnetic parts as well as the antisymmetric exchange contribution. All these factors give rise to a canted spin structure and subsequently to an uncompensated magnetic moment. Finally, we have demonstrated that the model perfect agrees with the experimental data on the static and dynamic susceptibility and hopefully incorporates the main physical interactions responsible for the SMC behavior of Co(H2L)(H2O) compound. ACKNOWLEDGEMENT 1-29 Financial support from the USA-Israel Binational Science Foundation (BSF Grant No. 2006498) is highly acknowledged. REFERENCES 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. D. Gatteschi, R. Sessoli and J. Villain, Molecular Nanomagnets; Oxford University Press: Oxford, 2006. D. Gatteschi, R. Sessoli, Quantum Tunneling of Magnetization and Related Phenomena in Molecular Materials, Angew. Chem. Int. Ed. 2003, 42, 268-297. A. Caneschi, D. Gatteschi, N. Lalioti, C. Sangregorio, R. Sessoli, G. Venturi, A. Vindigni, A. Rettori, M. G. Pini, M. A. Novak, Cobalt(II)-Nitronyl Nitroxide Chains as Molecular Magnetic Nanowires, Angew. Chem. Int. Ed. 2001, 40, 1760-1763. A. Caneschi, D. Gatteschi, N. Lalioti, R. Sessoli, L. Sorace, V. Tangoulis, A. Vindigni, Ising-Type Magnetic Anisotropy in a Cobalt(II) Nitronyl Nitroxide Compound: a Key to Understanding the Formation of Molecular Magnetic Nanowires, Chem-Eur. J. 2002, 8, 286-292. A. Caneschi, D. Gatteschi, N. Lalioti, C. Sangregorio, R. Sessoli, G. Venturi, A. Vindigni, A. Rettori, M. G. Pini, M. A. Novak, Glauber Slow Dynamics of the Magnetization in a Molecular Ising Chain, Europhys. Lett. 2002, 58, 771777. R. Clérac, H. Miyasaka, M. Yamashita, C. Coulon, Evidence for Single-Chain Magnet Behavior in a MnIII-NiII Chain Designed with High Spin Magnetic Units: A Route to High Temperature Metastable Magnets, J. Am. Chem. Soc. 2002, 124, 12837-12844. R. Lescouëzec, J. Vaissermann, C. Ruiz-Perez, F. Lloret, R. Carrasco, M. Julve, M. Verdaguer, Y. Dromzee, D. Gatteschi, W. Wernsdorfer, Cyanidebridged Iron(III)-Cobalt(II) double zigzag Ferromagnetic Chains: Two New Molecular Magnetic Nanowires, Angew. Chem. Int. Ed. Engl. 2003, 42, 14831486. H. Miyasaka, R. Clérac, K. Mizushima, K. Sugiura, M. Yamashita, W. Wernsdorfer, C. Coulon, [Mn2(saltmen)2Ni(pao)2(L)2](A)2 with L = Pyridine, 4-Picoline, 4-tert-Butylpyridine, N-Methylimidazole and A=ClO4, BF4 , PF6 , ReO4 : A Family of Single-Chain Magnets, Inorg. Chem. 2003, 42, 82038213. L. M. Toma, R. Lescouëzec, F. Lloret, M. Julve, J. Vaissermann, M. Verdaguer, Cyanide-bridged Fe(III)–Co(II) bis double zigzag chains with a slow relaxation of the magnetization, Chem. Commun. 2003, 1850 – 1851. T. F. Liu, D. Fu, S. Gao, Y.-Z. Zhang, H.-L. Sun, G. Su, Y.-J. Liu, An AzideBridged Homospin Single-Chain Magnet: [Co(2,2′-bithiazoline)(N3)2]n, J. Am. Chem. Soc. 2003, 125, 13976-13977. J.-P. Costes, J. M. Clemente-Juan, F. Dahan, J. Milon, Unprecedented (Cu2Ln)n Complexes (Ln=Gd3+, Tb3+): A New “Single Chain Magnet”, Inorg. Chem. 2004, 43, 8200-8202. M. Ferbinteanu, H. Miyasaka, W. Wernsdorfer, K. Nakata, K. Sugiura, M. Yamashita, C. Coulon, R. Clérac, Single-Chain Magnet (NEt4)[Mn2(5MeOsalen)2Fe(CN)6] Made of MnIII-FeIII-MnIII Trinuclear Single-Molecule Magnet with an ST=9/2 Spin Ground State, J. Am. Chem. Soc. 2005, 127, 3090-3099. 1-30 13. 14. 15. 16. 17. 18. 19. 20. 21. Z.-M. Sun, A. V. Prosvirin, H.-H. Zhao, J.-G. Mao, K. R. Dunbar, New type of single chain magnet based on spin canting in an antiferromagnetically coupled Co(II) chain, J. Appl. Phys. 2005, 97, 10B305. R. J. Glauber, Time dependent statistics of the Ising model, J. Math. Phys. 1963, 4, 294-307. A. V. Palii, S. M. Ostrovsky, S. I. Klokishner, O.S. Reu, Z.-M. Sun, A. V. Prosvirin, H.-H. Zhao, J.-G. Mao, K. R. Dunbar, Origin of the Single Chain Magnet Behavior of the Co(H2L)(H2O) Compound with a 1D Structure, J. Phys. Chem. A 2006, 110, 14003-14012. M. E. Lines, Magnetic Properties of CoCl2 and NiCl2, Phys. Rev. 1963, 131, 546-555. M. E. Lines, Orbital Angular Momentum in the Theory of Paramagnetic Clusters, J. Chem. Phys. 1971, 55, 2977-2984. A.V. Palii, B.S.Tsukerblat, E.Coronado, J.M. Clemente-Juan, J.J. BorrásAlmenar, Orbitally-dependent magnetic coupling between cobalt(II) ions: the problem of the magnetic anisotropy, J. Chem. Physics, 118 (2003) 5566-5581. D. A. Varshalovich, A. N. Moskalev, V. K. Khersonskii, Quantum Theory of Angular Momentum, World Scientific, Singapore, 1988. A. V. Palii, Ising limit for a pair of transition metal ions with unquenched orbital angular momenta and its relevance to single molecule magnets, Phys. Lett. A 2007, 365, 116-121. J. M. Yeomans, Statical Mechanics of Phase Transitions, Clarendon Press, Oxford, 1992. 1-31