CHEM 3364
advertisement

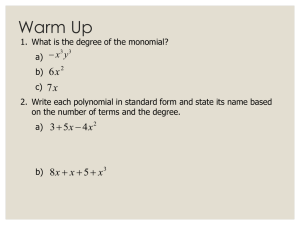
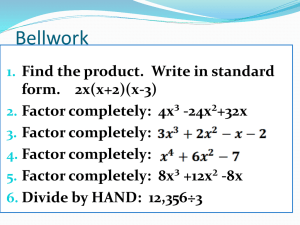
CHEM 3364 Special Functions Modeling Lab SPRING 2006 Harmonic Oscillator/Hermite Polynomials The solutions of the Schrödinger equation for the harmonic oscillator potential energy, V x the form: , y N Her , y e y2 2 1 2 where N 2 ! 1 2 kx , have 2 1 2 is the normalization constant and Her(, y) is the Hermite polynomial associated with the vibrational quantum number, . Hermite Polynomials We want to examine the behavior of the Hermite polynomials. Using Mathcad and Her(, y) as the Hermite polynomial function, plot the first ten Hermite polynomials. Using good style print these plots on a separate sheet of paper, i.e., conserve paper! 1. Are the polynomials finite? 2. Describe how the polynomials change with increasing quantum number. Harmonic Oscillator Wavefunctions Plot the first ten harmonic oscillator wavefunctions. Using good style, print these plots on a separate sheet of paper. 1. How are the wavefunction plots different than the polynomial plots? 2 2. Comparing the wavefunctions with their respective Hermite polynomials, why are wavefunctions finite? 3. Describe how the wavefunctions change with increasing quantum number. Harmonic Oscillator Probability Densities The probability density of a quantum state is found by taking the square of the wavefunction. Plot the first ten probability densities for the harmonic oscillator. Using good style, print these plots on a separate sheet of paper. 1. Describe how the probability density changes with increasing quantum number. 2. How are the probability densities different than the wavefunctions? 3 3. Based on the plot of the probability density, where within the potential well is the particle most likely to be when it’s in the ground state? 4. Based on the plot of the probability density, where within the potential well is the particle most likely to be when it’s in the 9th excited state? Orthonormality of Harmonic Oscillator Wavefunctions The harmonic oscillator wavefunctions are orthonormal. Using the wavefunction given above and the Calculus template in Mathcad, perform ten integrations. 1. Do five integrations on pairs of wavefunctions where the quantum numbers are different such as 1, y 2, y dy . Clearly state your results below. 2. Do five integrations on pairs of wavefunctions where the quantum numbers are the same such as 1, y 1, y dy . Clearly state your results below. 3. Are the Harmonic Oscillator wavefunctions orthonormal? 4 Hermite Polynomial Generating Function The Hermite polynomials can be generated as needed without referring to a table. The th Hermite polynomial is found by taking the th derivative of a simple function. This generating function is given below. 1 2 d 2 e y e y Her , y dy Find the first six Hermite polynomials by entering the generating function using the Calculus template in Mathcad (don’t type out the derivative, use the template!). Then click on the arrow in the Symbolic template followed by simplify in the Symbolic template. Note: Mathcad won’t take a zeroth derivative. Do the zeroth Hermite polynomial by hand. Her(0, y) = Her(1, y) = Her(2, y) = Her(3, y) = Her(4, y) = Her(5, y) = Now that you’re an expert, find the 16th Hermite polynomial. Her(16, y) = 5 Particle-on-a-sphere/Spherical Harmonics In this section we will be examining plots of the spherical harmonics. The spherical harmonics is the product of the associated Legendre polynomials for the “theta” portion of the wavefunction and the sinusoidal wave, eim, for the “phi” portion of the wavefunction. Because we want to visualize the wavefunctions in real, 3-dimensional space, we are going to use cos(m) for the “phi” portion. Finding the “phi” portion of the spherical is trivial; however, finding the associated Legendre polynomial is not. We use be using a generating function to find the polynomial. This generating function and the algorithm used with it is found on the Angular Wavefunction Supplement disk in the lab. Copy the algorithm to your worksheet. To visualize the spherical harmonics, we will need another algorithm on the Angular Wavefunction Supplement. Copy and paste the associated Legendre polynomial from the first algorithm into the appropriate spot in the second algorithm (the right hand side of f(,) =). Within the absolute value bars, be sure to include the appropriate “phi” function based on the m quantum number. Chemically Useful Spherical Harmonics 1. Make plots of the chemical useful spherical harmonics. Using good style, print these plots on a separate sheet of paper. s orbitals l = 0, m = 0 p orbitals l = 1, m = 0 l = 1, m = 1 d orbitals l = 2, m = 0 l = 2, m = 1 l = 2, m = 2 f orbitals l = 3, m = 0 l = 3, m = 1 l = 3, m = 2 l = 3, m = 3 Exotic Spherical Harmonics The spherical harmonics do have a pattern to their angular nodes; however, identifying that pattern is almost impossible by viewing the chemically useful spherical harmonics. Remember that the total number of angular nodes is given by the value of the “l” quantum number. However, if we do some deeper investigation, we can find a rule for the number of angular nodes in the “theta” direction and a rule for the number of angular nodes in the “phi” direction. Plot at least five “exotic” spherical harmonics to find these rules. E. g., plot some g orbitals, h orbitals, i orbitals, etc… Including a couple of plots where m = 0 and m = “l” should be helpful. Using good style, print these plots on a separate sheet of paper. 1. What is the rule for angular nodes in the “theta” direction based on the “l” and m quantum numbers? 2. What is the rule for angular nodes in the “phi” direction based on the “l” and m quantum numbers? 6 3. Find the total number of angular nodes by adding together the rule from question 1 and the rule from question 2. Radial Hydrogen Atom/Laguerre Polynomials The solutions of the radial portion of the Schrödinger equation that includes the Coulomb potential energy, 1 2 n 1 ! where N n ,n 2n n ! is the principal quantum number and “l” is the azimuthal quantum number. Lag n, , r are the associated Laguerre polynomials. To make the assignment simpler, we will study the basic Laguerre polynomials where “l” is always zero. Therefore for our purposes, we will not distinguish between 4s, 4p, etc… All of the orbitals studied will be s orbitals. This makes the wavefunction simpler, V r 2 1 Ze have the form: n, , r N n Lag n 1 , , r e 4 r r 2 1 r n 1 ! 2 2 n, r Lag n 1 , r e 2n n ! Laguerre Polynomials To examine the behavior of the Laguerre polynomials, we will use Mathcad and Lag(n, r) as the Laguerre polynomial function. Unfortunately Mathcad creates some confusion for us because it labels the Laguerre polynomials differently than we would like. We start our quantum numbers at 1. Mathcad begins labeling the Laguerre polynomials at 0. The n = 1 polynomial corresponds to Lag(0,r). The n = 2 polynomial corresponds to Lag(1,r). Plot the first ten Laguerre polynomials. Using good style, print these plots on a separate sheet of paper. 1. What happens to the radial wavefunctions when r < 0? 2. As the radius goes to infinity, does the Laguerre polynomial remain finite? 3. Describe how the polynomials change with increasing quantum number. 7 Radial Wavefunctions Plot the first five radial hydrogen atom wavefunctions. Using good style, print these plots on a separate sheet of paper. 1. What happens to the radial wavefunctions when r < 0? 2. For the hydrogen atom, what is the proper range for the r variable? 3. How are the wavefunction plots different than the polynomial plots? 4. Comparing the wavefunctions with their respective Laguerre polynomials, why are wavefunctions finite? 5. Describe how the wavefunctions change with increasing quantum number. 8 Radial Probability Densities The probability density of a quantum state is found by taking the square of the wavefunction. Plot the first five probability densities for the radial portion of the hydrogen atom. Using good style, print these plots on a separate sheet of paper. 1. Describe how the probability density changes with increasing quantum number. 2. How are the probability densities different than the wavefunctions? Radial Distribution Functions The radial distribution is the probability density multiplied by r2. Plot the first five radial distribution functions for the hydrogen atom. Using good style, print these plots on a separate piece of paper. 1. Describe how the radial distribution function changes with increasing quantum number. 2. How are the radial distribution functions different than the probability densities?