X-Rays in Medicine
advertisement

Diagnostic X-ray Imaging
1. Historical
8 Nov 1895 - x-rays discovered by Röntgen.
(Awarded 1901 Nobel prize for physics).
13 Jan 1896 - first clinical use. X-ray photograph
of a woman's hand produced in Birmingham.
During 1896 - used in high doses for the
treatment of Breast Cancer. Adverse affects to
patient and doctor were observed.
1913 - The first mammogram taken.
Technique essentially ignored for decades.
1917 - Radon presented the concept of
Computed Tomography (CT).
1972 - First clinical CT (or CAT) scanner
produced by Hounsfield. (Awarded 1979
Nobel Prize for Medicine.)
1
2. Production of x-rays – Simple X-ray generator
Electrons accelerated through large potential
before hitting the anode target.
Electron K.E. partially converted into E.M.
radiation.
X-rays emitted in all directions
greatest intensity at right angles to the
electron beam.
Conversion process is very inefficient: 99%
conversion to heat.
Anode is usually rotated at high speed to help
heat dissipation.
2
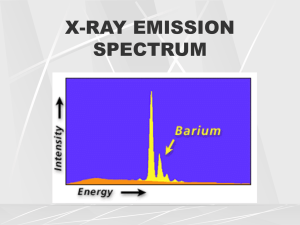
3. X-ray spectra
Firstly, the electron deceleration which occurs close to
nuclei in the target produces a wide continuous
spectrum of x-rays (Bremsstrahlung or 'white x-rays')
The variation in the intensity of the emitted x-ray
photons as a function of photon energy can be
explained as follows. First, imagine a very thin anode,
and consider the production of X-rays, not the X-rays
that finally emerge. Consider the intensity of X-rays
produced in a small energy range E to E+dE, this will be
equal to the number of photons/m2/sec multiplied by the
photon energy E. Fewer high energy photons are
produced but their energy is higher and the product is
constant. Thus for a thin anode we would have (a)
overleaf.
3
A thick anode may now be thought of as composed of a
large number of thin layers. Each will produce a similar
distribution to that shown in (a), but the maximum
photon energy will gradually be reduced because the
incident electrons lose energy as they penetrate the
anode material. Thus the composite picture for X-ray
production might be as shown in (b).
However, before the X-rays emerge, the intensity
distribution will be modified in two ways. First, X-rays
produced deep in the anode will be attenuated in
reaching the surface of the anode and secondly X-rays
will be attenuated in penetrating the window of the X-ray
tube. Both processes reduce the intensity of the low
energy radiation more than that of the higher energies
so the result is the solid curve shown in (c).
In the absence of further filtration (see later) the X-ray
energy corresponding to maximum intensity will be
about one third of the highest energy X-ray photons.
4
Superimposed on the continuous spectrum are sharp
characteristic lines. Caused by ejection of K and L shell
electrons followed by outer shell electrons filling the
vacancy which was created.
5
3.1 Effect of tube voltage on x-ray spectrum
Maximum x-ray energy corresponds to full
deceleration of the electron:
Emax = eVmax = hfmax = hc / λmin
so,
λmin = hc / (eVmax)
E(keV)=1.24/ λ(nm) (E=13keV, λ≈0.1nm)
As tube voltage V is increased:
spread of wavelengths increases
intensity increases (total intensity V2)
peak in intensity shifts to higher energy
Effect of tube voltage on x-ray spectrum
6
3.2 Effect of tube current on x-ray spectrum
As the tube current i is increased:
rate of production of electrons at the cathode
is increased
intensity increases (total intensity i)
maximum energy remains unchanged
intensity profile remains the same
Effect of tube current on x-ray spectrum
7
3.3 Effect of target material on x-ray spectrum
Changing the target material changes the
atomic number, Z:
x-ray intensity changes - the probability of a
collision and so intensity Z
changes the characteristic lines
Tungsten (Z = 74) is almost always used as a
target material:
reasonably high Z
high melting point (3650 K) - vital because of heat
production
8
4. Attenuation of x-rays
As an x-ray beam propagates, photons are
scattered out of the beam: hence the beam is
attenuated.
Number of photons scattered per unit beam area in
distance Δx is ΔΦ = ΦσNΔx
N is no. of nuclei per unit volume,
σ is the "scattering cross-section" - fractional beam area
that interacts
Φ is the fluence (no. of photons per unit area).
Intensity of x-ray beam I is rate of energy incident per unit
area, so is proportional to Φ.
Change in intensity over distance Δx is ΔI = -lσNΔx.
9
Integrating gives logel = -σNx + k.
Let l0 be intensity when x = 0, so k = loge l0
So
l = l0e-σNx
Define the linear attenuation coefficient as μ =σN,
so
I = I0e-μx.
N and μ, both related to the density ρ of the medium:
N = NAρ/M ,
NA is Avogadro's number and M is the molecular or
atomic mass,
So μ = σNAρ/M
So high density media yield high μ values.
μ also depends on Z through σ and M.
Mass attenuation coefficient, μ/ρ = σNA/M, depends on Z
(through M) and photon energy (through σ ). σ also
depends on Z
10
4.1 Attenuation mechanisms
Attenuation = Absorption + Scatter
Dependent on incident photon energy (E).
Medical imaging requires best contrast and least
damage.
(i) Simple scattering
photon energy << electron binding
elastic collision
μ / ρ Z2 / E
When X-rays pass close to an atom, they can excite
electron vibrations. The process is one of
resonance, such that the electron vibrates at the
same frequency as the incident X-ray photon. This
is an unstable state and the electron quickly reradiates this energy in all directions at exactly the
same frequency as the incident photons. The
process is one of scatter and attenuation without
absorption. The electrons that vibrate in this way
must remain bound to their nuclei – thus the
process involves bound electrons. Binding energy
increases with Z.
11
(ii) Photoelectric effect
photon energy > binding energy
all photon energy given to an inner electron which
is ejected. Characteristic x-ray emitted
ejected electron ionizes atoms along its path until
its kinetic energy is dissipated
μ / ρ Z3 / E3 (crude approximation)
12
(iii) Compton Scattering
photon energy >> binding energy
photon energy transferred to an outer 'free'
electron which is ejected
ejected electron energy depends on angle
through which incident photon scattered (size of
arrow in figure below)
photon continues with reduced energy
electron dissipates energy by ionizing atoms
along its path
μ / ρ independent of Z and falls slowly with E
13
(iv) Pair production
Very high incident photon energies
(>1.02 MeV)
Pair of anti-particles (electron+positron)
formed in nuclear coulomb field
μ / ρ Z2 and rises very slowly with E
Thus overall attenuation is a combination of (i) to
(iv) thus for a path length x through an object
I = I0 e-μ1 x e-μ2 x e-μ3 x e-μ4 x
I = I0 e -(μ1 +μ2 +μ3 +μ4) x
14
4.2 Relative importance for medical imaging
Left – Relative attenuation coefficients between materials.
Right – Attenuation mechanisms in water (similar to soft tissue)
Contrast decreased as incident photon energy is
increased
Best contrast: photoelectric effect (Z3)
Scattering causes image blurring
High energy attenuation causes damage (through
ionization and heating)
20 - 100 keV used for diagnostic radiology
(photoelectric effect and Compton scattering)
> 100 keV used when contrast between bone and
surrounding structure not required (e.g. imaging lungs)
High Z elements may be used to improve contrast
e.g. injected NaI to investigate circulatory system
or barium sulphate in gastro-intestinal system
15
16
17
18
4.3 Filtration of x-ray sources
Want maximum intensity in useful energy
range.
high energy extreme controlled by varying tube
voltage
Low energy photons absorbed in or near skin:
no effect on contrast just an increased dose
Iow energy: photoelectric effect dominates
AI commonly used as a filter: μ/ρ
proportional to Z3/E3 so low energies
preferentially absorbed. (Al 1-3mm thick)
overall reduction of x-ray intensity; peak shifted
to higher energy ('hardening' beam)
19
4.4 Dose and exposure
X-rays cause damage to tissue through
ionization, so x-ray dose must be kept small.
Dose defined as:
D = Energy absorbed in mass
mass
Units: Gray (Gy); 1 Gy = 1 J kg-1
Old unit: rad (radiation absorbed dose)
1 Gy = 100 rad
Absorbed dose difficult to measure, so often use
measured Exposure to determine dose.
An Exposure of 1 C kg-1 in air produces 1/e
electrons per kg of air (C Coulomb).
Dose (Gy) f x Exposure (C kg-1) [f = 34 in air]
In soft tissue f = 34 to 40, higher for bone.
Depends on photon energy.
Old unit: Röntgen (R); 1 C kg-1 = 3876 R.
20
5. X-ray photographs
Advantages:
Cheap
Equipment easy to maintain and use
Readily available
Relatively safe
21
5.1 Blurring
(i) Scattering
Scattering leads to loss of spatial resolution.
scattered x-rays eliminated using a grid of lead
strips between patient and film.
strips angled to receive only direct beam
strips continually moved to avoid being
imaged
22
(ii) Patient movement
Involuntary movement of organs can lead to
blurring
overcome by using short exposure times.
to maintain exposure must use high
intensities (increased dose rate)
Often image intensifier used instead.
Fluorescent screens placed either side of the film
(e.g. zinc cadmium sulphide doped with silver)
Metal backing plate used to avoid
backscatter and x-ray leakage
10-40 intensification factor possible
Some definition lost by diffusion of
fluorescent light
23
(iii) Focal spot size
penumbra shadow formed if large effective focal spot
used (a)
angling anode gives smaller effective focus (penumbra
decreased) (b)
patient-film distance also minimised
24
5.2 Limitations
Features behind e.g. bones, are difficult to
Image
photograph 2-D projection of a 3-D object
cannot establish where the feature is in the
path
the contrast is a sum of attenuations in the
path (so possibility of ghost objects)
Can get around these problems by taking two
projections
25
6. Linear Tomography
Tomography is a method of viewing a slice through
the body. (Greek Tomos - slice)
source and film moved in opposite directions at the
same speed
any point in the image plane will appear in the same
position on the film
all other points image at different positions on film as
source and film are moved and thus image blurred
(e.g. point closer to source than image plane - dotted
lines indicate path of X-ray photons)
depth resolution of approx. 1 mm possible
26
6.1 Selection of image plane
Consider a rod connecting source (S) and film cassette
(F), with pivot, P, at a variable point as in diagram. The
slice through body in focus changes as pivot moves up
and down. The thickness of the cut is controlled by the
size of tomographic angle θ. The greater the value of θ
the thinner the slice will be. This is because even
structures very close to the pivot are blurred, since their
image moves significantly on the film. If θ=0 it is a
conventional radiograph.
If the movement runs parallel to an elongated body
structure (e.g. femur) there will be comparatively little
blurring of the long edges even when not in the pivot
plane. A circular instead of linear movement can help in
such cases.
27
7. Computed Axial Tomography (CT or CAT)
Radon showed that it is possible to build an image
of an unknown object given an infinite number of
projections through object
CAT relies on obtaining many projections from
different axial positions.
Each ray in a linear projection provides an
intensity data point that depends on the
cumulated absorption coefficient.
Distance between projections ~0.5 mm
28
7.1 Tomographic reconstruction
Main type of tomographic reconstruction is
filtered back projection.
Integration of absorption coefficient (μ) over path,
must be 'undone'.
achieved by 'smearing' μ, over the path
grid of pixels created, and value of μ from ray
added to value of each pixel on line - repeated
for each ray and each projection
pixel values are divided by number of
values of μ added, and converted to a grayscale for display
each pixel actually a volume element
(voxel), typically 1 to 10 mm deep
29
30
31
32
33
34
35
7.2 Scanner development
Scanner design continually modified to improve
image quality and increase scan speed.
(i) First generation
one ray source and one detector
source and detector moved linearly to obtain line
scan
source and detector rotated in 1° intervals, and
linear scan repeated. Repeated for 180°.
Solid state scintillation, or argon ionization
chamber detector used.
Advantages:
Excellent scatter rejection
Disadvantages:
Long scan time (up to 5 mins)
36
(ii) Second generation
narrow fan source beam (10°) used.
detector is a linear array of 30 detectors
source and detector array are moved linearly
source and detector are rotated in 10°
intervals to cover the 180° arc.
Advantages:
Much shorter scan times (typically 20 sec)
3 times as many measurements, so
improved image resolution.
Disadvantages:
Decrease in scatter rejection
Each detector must be gain-balanced.
37
(iii) Third generation
Larger fan angle to cover whole body.
750 detectors arranged along the circular arc
source and detector array rotated to take a
number of scans
Advantages:
No translation and many more detectors, so very
fast scans (typically 5 sec).
Disadvantages:
Much increased cost
750 rotating detectors mean cable problems
Detectors must be balanced
38
(iv) Fourth generation
Complete ring of detectors (1000 detectors)
Wide fan x-ray source rotated
Advantages:
Only source cabling needs to be rotated.
Detectors are self-balancing: at some time each
detector views a beam that does not pass
through the body.
Stable x-ray source required
Increased scan speed (1 second)
Disadvantages:
Worse scatter rejection: each detector sees all
beam directions.
X-ray source must rotate on a ring closer to
patient: decreased resolution (higher
magnification). Can be overcome by bending
detector ring out of the way (nutation).
39
40
(v) Fifth generation (cine-CT)
Developed to achieve very fast scan times to
monitor pulsating organs (e.g. heart or liver)
No moving parts
Complete ring of detectors (as in 4th
generation)
X-ray source: ring of tungsten around the patient
used as target for an electron beam, which is
directed by magnetic field.
Advantages:
50 ms scan times.
Disadvantages:
Very low intensity x-rays, therefore poorer quality
images.
41
42
43
44
Here the average attenuation along a particular direction
is put in every cell along that direction. If there is already
a value in the cell the new average for the new direction
is added to the existing value. Thus the cells in the first
column each start with the average vertical attenuation
per cell along the first column (i.e. 20/3) followed by the
average attenuations in the horizontal direction (20/3 for
first row) and diagonal directions (25/3 for top left to
bottom right cells and 5/1 for diagonal through just top left
hand cell). The resulting attenuation coefficients for each
cell calculated in this way are ringed.
45