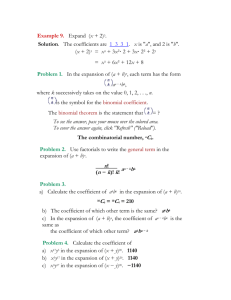
Theory:
advertisement

LIQUID-LIQUID EXTRACTION FORMAL REPORT THOMAS SALERNO GROUP MEMBERS: GREGORY ROTHSCHING AN DU i ABSTRACT In order to determine the correlation of the mass transfer rate, overall mass transfer coefficient, and terminal velocity with diameter during single drop microextraction of acetic acid from toluene by water, this experiment was designed to educate engineers in this practice. It is also the intention of this paper to reveal the accuracy of predictive equations used to design extraction columns. To achieve these objectives, my fellow teammates and I placed our toluene phase, of .776 M acetic acid, into a large graduated cylinder. From a syringe suspended above the column, spherical drops of our aqueous phase, made up of .01M NaOH and phenolphthalein, were released at diameters of 3.5, 4.0, and 5.1mm. By recording the time for each drop to traverse .213m of the column, we determined that its terminal velocity increased with increasing diameter with values of 9.5, 10.3, 11.9 cm/sec. Furthermore, the time recorded for each drop to turn clear (i.e. the entire concentration of NaOH neutralized), we were able to conclude the mass transfer rate increased with increasing diameters with values of 6.0, 7.9, 12.9 e-8 mole/sec. By mixing equal amounts of toluene and water with acetic acid and titrating each phase, we ascertained that the equilibrium distribution coefficient of our solution is 10.63±1. Thereby, we were able to declare the equilibrium concentration of aqueous acetic acid at the surface of our drop to be 8.25M. This data was inputted into a mass transfer form of Newton’s Law of Cooling, N HAc K * A * C * CHAc ,Wat , to calculate the overall mass transfer coefficient to decrease with our increasing diameters with values of 1.95, 1.92, 1.87 e-7 m/sec. Because the terminal velocities measured were an average of 22% below those predicted for rigid spheres, and the drops were observed to fall in a helix pattern, we determined that the drops experienced oscillations on its track down the column. By using the model of Handlos and Baron, the overall mass transfer coefficients could be predicted within errors of only 1.5%. with an experimentally determined instability factor of 113.3 as suggested by Henschke and Pfenning. In summary, this report has established that an increase in diameter of a fallen drop will cause an increase in its terminal velocity, a decrease in its overall mass transfer coefficient (increase in inside mass transfer coefficient but overriding decrease in outside mass transfer coefficient), and an increase in its mass transfer rate. This trend indicates that the toluene (outside) phase is the controlling resistance to mass transfer, and that the surface area of the drop is the controlling factor for the mass transfer rate. We also verified that the trends of our state variables were predicted, but that experimental measurements were required for prediction of absolute numbers. When designing an extraction column, these results are most significant for the chemical engineer. ii TABLE OF CONTENTS ABSTRACT ................................................................................................................................................. II TABLE OF CONTENTS ........................................................................................................................... III INTRODUCTION: ....................................................................................................................................... 1 THEORY: ..................................................................................................................................................... 5 EXPERIMENTAL: ....................................................................................................................................... 5 Introduction: ........................................................................................................................................ 5 Figure 1: Addition of water phase into column as spherical drops. .............................................................. 5 Figure 2: Extraction of HAc from toluene phase by water phase. ................................................................ 6 Terminal Velocity:................................................................................................................................ 6 Figure 3: Schematic of forces acting on drop. ............................................................................................... 6 Molar Rate of Transfer: ....................................................................................................................... 7 Equilibrium Distribution Coefficient: ................................................................................................. 9 Overall Mass Transfer Coefficient: ..................................................................................................... 9 THEORETICAL:.........................................................................................................................................11 Introduction: .......................................................................................................................................11 Figure 4: Concentration Profile of out two phase liquid-liquid system. ...................................................... 12 Dimensional Analysis .........................................................................................................................13 Table 1: Important variables in our liquid-liquid extraction system. .......................................................... 13 Terminal Velocity:...............................................................................................................................15 Figure 3: Schematic of forces acting on drop. ............................................................................................. 15 Mass Transfer Coefficient Outside of Drop: ......................................................................................17 Mass Transfer Coefficient Inside of Drop..........................................................................................19 Figure 4: Circulation patterns in the drop. .................................................................................................. 20 Figure 5: Drop with tori superimposed on the right. ................................................................................... 21 Mass Transfer Coefficient Inside and Outside of Drop (Fudge Factor)...........................................26 Overall Mass Transfer Coefficient: ....................................................................................................27 Figure 6: Graphical representation of concentration difference driving force. .......................................... 28 EQUIPMENT AND PROCEDURE: .........................................................................................................31 PART 1: CALCULATING VELOCITY AND MASS TRANSFER RATE ...........................................................31 Figure 7: Schematic of graduate cylinder used to hold toluene phase. ....................................................... 31 Figure 8: Schematic of syringe, plunger, sample pot, and syringe pump Model 341. ................................ 32 PART 2: TITRATION .................................................................................................................................34 Figure 9: Schematic of titration equipment. ................................................................................................ 34 RESULTS AND DISCUSSIONS: ..............................................................................................................38 TERMINAL VELOCITY: ............................................................................................................................38 Table 2: Average times recorded for drop to fall .213m in the column. ...................................................... 39 Figure 10: Terminal velocity of the drop (Experimental and Predicted) as a function of drop diameter .. 39 Table 3: Measured and Predicted Terminal Velocities of Drop ................................................................... 40 CONCENTRATION OF ACETIC ACID IN TOLUENE: ..................................................................................41 Table 4: Concentration of HAc in the toluene phase. .................................................................................. 42 DISTRIBUTION COEFFICIENT: .................................................................................................................42 Table 5: Experimentally determined distribution coefficient. ...................................................................... 43 Table 6: Experimental and Predicted distribution coefficients. ................................................................... 44 OVERALL MASS TRANSFER RATE: .........................................................................................................44 Figure 11: Molar Transfer Rate as a function of drop diameter. ................................................................. 46 OVERALL MASS TRANSFER COEFFICIENT: ..............................................................................................46 Figure 12: Overall Mass Transfer Coefficient as a function of drop diameter ........................................... 49 Figure 13: Graph of Kpred versus Kexp to determine CIP ........................................................................... 52 Figure 14: Fudge Factor predicted and experimental overall mass transfer coefficient as a function of drop diameter.................................................................................................................................................. 53 INDIVIDUAL MASS TRANSFER COEFFICIENTS: .........................................................................................53 iii Figure15: Individual mass transfer coefficients as a function of drop diameter. ....................................... 54 REVISITING THE OVERALL MASS TRANSFER RATE: .............................................................................55 Table 7: Percentage Growth of the drop’s surface area and overall mass transfer coefficient. .................. 56 CONCLUSIONS: ........................................................................................................................................57 RECOMMENDATIONS: ...........................................................................................................................63 NOMENCLATURE: ...................................................................................................................................64 ABBREVIATIONS:......................................................................................................................................64 DIMENSIONLESS NUMBERS: ....................................................................................................................64 VARIABLES:..............................................................................................................................................64 REFERENCES: ...........................................................................................................................................66 APPENDIX: .................................................................................................................................................67 RAW DATA: ..............................................................................................................................................67 Table 8: Raw Data, Week 1, Large Syringe .................................................................................................. 68 Table 9: Raw Data, Week 1, Medium Syringe .............................................................................................. 69 Table 10: Raw Data, Week 1, Small Syringe ................................................................................................ 70 Table 11: Raw Data, Week 2, Titrations ....................................................................................................... 71 SAMPLE CALCULATIONS: ........................................................................................................................72 Experimental: ......................................................................................................................................72 Theoretical: .........................................................................................................................................78 Figure 13: Graph of Kpred versus Kexp to determine CIP ........................................................................... 83 iv INTRODUCTION: A relatively cheap method to separate a two component mixture is through the process of liquid-liquid extraction. This particular unit operation is of major importance to the chemical engineer and is the subject of this report. Every research project needs a starting point. So let’s begin by supposing a specific liquid reactant, R, is required for your process. In order to obtain your reactant, there are two possible paths that may be chosen. First, is to purchase this product in its purest form which could prove to be quite expensive. Second, is to derive it indirectly from your own reaction in a non-pure state. It follows that there will be present some impurity, A, that must be removed in order for it to be used in your process. Based on the components themselves, there are a number of approaches to accomplish the removal of impurities from a product. One method is by distillation. However, this method requires both substances to be thermally stable over a wide range of temperatures, and have a substantial difference in their relative volatilities. But, even if these criteria are met, distillation, itself, incurs substantial operating expenses because of the requisite heat exchangers required for a condenser and a reboiler. A more attractive option is to separate the impurity through liquid – liquid extraction (or gas-liquid extraction if more applicable). This method involves using a solvent, E, which is immiscible with the R phase, and has a greater affinity for the impurity A. Thus, if an equilibrium stage extraction unit is set up, we can transfer the impurity A out of our desired reactant R, and into our solvent E. The main advantage in using this technique is the lack of an energy expense, because these phases do not have to be boiled nor condensed. Also, the lack of heating allows us to perform this procedure on many components even if they are temperature sensitive. By examining current industry processes, it is quite evident that this method is the most attractive by its use in a wide variety of applications. For example, liquid – 1 liquid extraction is used in the production of penicillin and for the separation of aromatic products from other mixed hydrocarbons that come out of the efflux of the reactor, etc. Another chic characteristic of this process is the generality of the equations used to design the unit. For example, in this experiment, we will study the extraction of acetic acid from toluene by water. The correlations and theories developed from this experiment will be applicable to the above mentioned extractions and many other extraction processes that may arise in the chemical engineer’s course of action. To reduce costs and increase accuracy in the designing of industrial extraction columns, engineers often necessitate similar experiments to be carried out on a laboratory-scale, rather than a pilot-plant scale. Therefore, it is increasingly important to be able to develop theoretical models that transfer mass between single drops and a stagnant continuous phase and enhance the capability to adapt this to industrial scale columns. In order to achieve this goal, mathematical models are needed for the description of mass transfer and drop motion for a single drop, and the effect of these parameters on drop diameter. This report is limited to satisfying the modeling of mass transfer from a single drop and discussing the application of these models in industrial processes. Thus, leaving the actual math involved in this last part as a recommendation for further analysis. More specifically, this experiment will study chemical extraction (mass transfer aided by the push of the reactants to react to form product), as opposed to mechanical extraction (mass transfer driven solely by the impurity’s greater affinity for the E phase relative to the R phase), because the water phase will contain .01M NaOH as well as several drops of phenolphthalein. In order to make experimental measurements easier and more accurate without requiring expensive equipment, this basic concentration of the water phase was utilized. In our particular setup, as the acetic acid diffuses into the water phase, it will react with the NaOH and neutralize it, thus making the solution less basic and consequentially less red. This, also, has the effect of keeping the concentration of acetic acid in the water phase drop at zero for the entire time of experimental measurement. Once the known amount of NaOH has been completely neutralized, 2 the drop will appear clear, and we will be able to calculate the exact amount of time required to diffuse in a known amount of acetic acid. Note, that chemical extraction can differ in its analysis from mechanical extraction in three major areas. First, the concentration of A in the E phase will always remain zero because A will react immediately to form product. Second, the diffusion path of the impurity A will be reduced, however, as this report will demonstrate, the diffusion path will be unimportant due to the predicted oscillations occurring which help to keep a uniform mix in the drop. Finally, chemical extraction will cause the mass transfer to be irreversible, due to the A disappearing upon diffusing into the E phase. Even though chemical extraction is modeled exactly like that of mechanical extraction, there is one difference being the extra effectiveness of the driving force in driving mass transfer due to the reactants wanting to form product. This extra effectiveness will be modeled in the testing by a simple experimental constant, which will be absorbed into the instability constant to be discussed later. This analysis by a constant is allowed because of the oscillations occurring in the drop, a theory that will be discussed later. Thus the procedures, equations, and theories from this type of extraction presented in this report can be equally applicable to mechanical extraction. Included in this procedure, will be the use of an aqueous solution (AQ) consisting of .01M NaOH and phenolphthalein (pink solution), introduced as spherical drops into an organic solution (ORG) of known molarity of HAc (to be determined later). The main focus of this experiment is to determine the effect our independent variable (drop diameter, D) will have on our dependent variables: the drops terminal velocity (v), mass transfer rate (Na), and mass transfer coefficient (Ki). Furthermore, as a result of this experiment, we will be able to determine the distribution coefficient (m) for acetic acid between T and W and reveal the accuracy of the theoretically developed predictive equations. Throughout the first week, a glass cylinder was filled with an unknown molarity of the ORG. With the use of a cut syringe, a large (roughly .5cm diameter) drop of the AQ was inserted at the top of the cylinder. The molarity of the ORG was adjusted 3 such as to make the drop turn clear approximately ¾’s of the way down the column. Next, we inserted varying diameters of the AQ, namely .5, .45, and .4cm. For each diameter, 10 drops were used to determine the amount of time needed to travel a certain distance (finding v); and another 10 drops were used to determine the time needed to become clear (neutralized). With this data, my lab partners and I calculated the mass transfer rate to be ( N a Voldrop CNaOH / Time ). In the second week, NaOH was used to make my ORG from the cylinder basic, thus, ensuring that all of the acetic acid present in the solution had been neutralized, and back titrated with HCl to find the molarity of the toluene, CA,T. Then, by mixing 50ml of both T and W with a given amount of HAc (calculated to achieve the same CA,T as found in the column) and titrating each phase, we found the distribution coefficient for HAc in T and W at this concentration, m C A,W / C A,T . Upon completion of this phase in the experiment, we were able to determine the effect diameter had on the overall mass transfer coefficient, the mass transfer rate, and the terminal velocity of the drop. Also with this resulting data, the equilibrium distribution coefficient of acetic acid in water and toluene was calculated. This data was then compared to developed models of Handlos and Baron, with adjustments made by Henschke and Pfenning to prove the accuracy in predicting not only the trends produced but also the absolute numbers. 4 THEORY: Experimental: Introduction: The Falling Drop Experiment studies the microextraction of Acetic Acid (HAc) from toluene (Tol) by water (Wat). The term microextraction signifies that this experiment does not involve continuous streams of both phases. Rather, the toluene (or organic) phase has a certain concentration of acetic acid in it, namely, CHAc ,Tol , and it remains uniformly mixed and stagnant in a large graduated cylinder. Wat is then inserted as spherical drops, one at a time into the cylinder as shown in Figure 1. Vo lu metric Flo w: mL per Minute Vo lu metric Flo w: mL per Hour Figure 1: Addition of water phase into column as spherical drops. The extraction of HAc occurs via a transfer from the bulk (toluene) phase, into a small sphere of water, as can be seen in Figure 2. 5 NaOH Toluene Phase Wate r Phase Neutralization Reaction HAc Mass Transfer Product Figure 2: Extraction of HAc from toluene phase by water phase. Terminal Velocity: Once the sphere of water enters the large volume of toluene, it will have three forces acting on it. These are described as the force of gravity which pulls the sphere vertically down the column, the buoyancy force which acts to push the sphere up the column, and a drag force which acts in the direction opposite velocity. This situation is illustrated in Figure 3. FDrag FBouyancy FDrag Velocity FGravity Figure 3: Schematic of forces acting on drop. It is important to note from Figure 3 that we have drawn the drag force as pushing the spherical drop up. This only happens when the velocity of the particle is down, which we know to be the case. Our math, however, will take this unknown direction of drag force into account. 6 We, then, apply Newton’s Law, which states that the sum of the forces is equal to the mass of the particle, multiplied by its acceleration. (1) M* C v v surrounding fluid Aprojected dv M M * agravity surrounding fluid agravity D dt drop 2 In this equation, M is the mass of the drop in kilograms, v is the velocity of the drop in meters per second, agravity is the acceleration due to gravity in meters per second squared, surrounding fluid and drop are the densities of the bulk surrounding fluid and of the drop respectively, both in kg per meter cubed, CD is the drag coefficient for a sphere, and Aprojected is the projected area of the sphere which is the area of a circle. However, for all practical purposes, the drop, upon entering the toluene phase will immediately reach a terminal velocity, vT, at which dv 0 , and thus will travel dt with a constant velocity the entire way down the column. In order to experimentally obtain this terminal velocity, we have to measure the time it takes for the particle to travel a certain distance. Subsequently, Equation 2 is used to obtain vT. (2) vT distance traveled by particle . time Molar Rate of Transfer: In order to measure the rate of extraction, we are going to perform an in-situ titration. 1M NaOH and a sufficient amount of phenolphthalein are used in the aqueous phase creating a dark red solution. Since the Wat phase has a greater affinity for HAc than the Tol phase, as the Wat drop passes down the column it will continuously be extracting HAc into it. However, because of the presence of the NaOH in the Wat, the HAc will react according to: 7 (3) CH 3CH 2COOH (aq) NaOH (aq) H 2O(l ) CH 3CH 2COO Na (aq ) Because of the high equilibrium coefficient of this reaction, the HAc will immediately reach and disappear. Thus, as long as there is still NaOH present in the water, the concentration of HAc in the water, C HAc ,Wat , will be assumed zero. As the NaOH is being neutralized by the diffusing HAc, the phenolphthalein will have a less basic atmosphere to cause a red color, thus, the deep red color will fade to pink and eventually become clear. With our current equipment, the rate of this change is impossible to quantitatively measure. However, we can measure the time it takes for all of the reddish/pinkish color to disappear, and, thus, be able to measure the time it takes to neutralize all of the NaOH present. We are able to measure the amount of NaOH which had to be neutralized from Equation 4 because we have set the original concentration of NaOH present in the water drop. (4) 4 3 Vdrop rdrop 3 nNaOH ,initial Vdrop CNaOH ,initial By taking an average of the volume of 50 drops from the same syringe, we can estimate Vdrop . Then, from Equation 1, we determine that one mole of HAc must have been extracted for every mole of NaOH that has been neutralized, and we can write, (5) nHAc ,extracted nNaOH ,neutralized . Next, we can measure the experimental molar transfer rate by dividing the amount of HAc transferred by the time it took to fully neutralize the drop of water, as is shown in Equation 6. 8 (6) n N HAc HAc ,extracted time Equilibrium Distribution Coefficient: Finding the experimental distribution coefficient of HAc between Wat and Tol is easily accomplished after examining what this term represents. The distribution coefficient reveals how the acetic acid will transfer itself when it is present in a mixture of toluene and water and is defined as, (7) C m HAc ,Wat C HAc ,Tol . Thus, we could mix an equal amount of Tol and Wat with a certain amount of acetic acid. Because these two phases are immiscible, we can easily separate these phases with a separatory funnel. To find out the concentration of HAc in each phase, we would titrate each.. Then by using Equation 7, we will find the distribution coefficient. However, the distribution coefficient, m, is concentration dependant. Thus, we need to perform this procedure such that the CHAc ,Tol established, matches that found in the column. The formula for this is outlined in the procedure section of this report. Overall Mass Transfer Coefficient: The next step in the analysis of this experiment is to fit the data into a simple rate law. We can develop such a law by analyzing the physical situation occurring. As described earlier, once the sphere has entered the toluene phase, it will travel down at an approximately constant velocity of vT. The sphere’s presence will introduce a concentration gradient into the system because the bulk atmosphere has a concentration of HAc of CHAc ,Tol , while the inside of the sphere will initially have a concentration of HAc of CHAc ,Wat 0 . Thus, there exists a driving force to 9 balance out this concentration gradient and cause transfer of HAc across the interface of the bulk toluene phase and the drop. Because the drop in the toluene is moving, relative to the drop, so too is the interface for this transfer, therefore we have convective mass transfer taking place. We can model this transfer by deriving an expression from Newton’s Law of Cooling shown in Equation 8. (8) Rate of Transfer = Constant* Driving Force The rate of transfer we are looking for is the molar rate of transfer of acetic acid. We have previously defined the driving force for this transfer, as being the concentration difference at the surface of the drop and the concentration at the center of the drop. Subsequently, we can write Equation 8 in a more useful form as, (9) N HAc K * A * C * CHAc ,Wat In this equation, we have defined K as the overall mass transfer coefficient, A is the surface are of the drop, C* as the concentration of HAc at the surface of the drop and C HAc ,Wat as the concentration of HAc present in the drop. We can simplify this expression by ,again, noting that because of the high equilibrium constant of Equation 3, the concentration of HAc present in the drop will be zero at all times before total neutralization of the NaOH present in the water. We can write this as, (10) N HAc K * A * C * All that remains is to find K, the overall mass transfer coefficient, is an expression to find C*. This can be accomplished by assuming the diffusion of HAc into the drop occurs via diffusion at temporary stagnant points at the surface of the drop. At this moment in time, the molecules of water at the surface will be in 10 equilibrium with the toluene phase. Thus, the concentration of HAc in these molecules of water will be such that they are in equilibrium with the surrounding molecules of toluene at a known concentration of CHAc ,Tol . Hence, we are able to use Equation 7 to write, (11) K N HAc m * CHAc,Tol * A We can find K through Equation 11 because all of the variables are known. Theoretical: Introduction: From a purely mathematical perspective, it is our intention to derive an expression for the rate of mass transfer. This expression would allow us to predict transfer rates from theory without always having to run laboratory experiments. Because of its purely mathematical basis, this development would be practical for formulating immediate predictions and permitting us to see which factors have a strong impact on the mass transfer by allowing us to see trends in the data. As described by the derived Newton’s law for mass transfer, Equations 8 and 9, the rate of mass transfer in an extraction process will depend on three things, the area of contact, the effective driving force, and the transfer coefficient. As explained previously, we can write this equation for our microextraction process as Equation 10, reproduced below, (10) N HAc K * A * C * Where N HAc is the molar rate of transfer of HAc, K is the overall heat transfer coefficient, A is the surface area of the drop, and C* is the concentration of HAc 11 at the surface of the drop. In this equation, we can easily calculate the surface area of the drop through (12) 2 A 4* * rdrop And, as stated before, we can use Equation 11, reproduced below, to calculate C*, (11) K N HAc . m * CHAc,Tol * A Thus, in order to find the molar rate of transfer, we only need to develop a method to determine K, the overall mass transfer coefficient, from theory. This process, however, is not so straightforward. The difficulty becomes clear when you analyze the interface of two phases in equilibrium. For our situation, we have toluene and water striving to obtain equilibrium, at the surface of the aqueous drop, in the transfer of acetic acid, shown in Figure 4. Figure 4: Concentration Profile of out two phase liquid-liquid system. It is evident from Figure 4, that each phase will develop its own smooth concentration gradient within itself; however, these two concentration gradients will not be continuous at the surface. This is due to non-proportionate affinities among the two phases for acetic acid. In our situation, the acetic acid has a greater chemical affinity for the water and therefore in order to obtain chemical 12 equilibrium by equating the chemical potentials of the two phases, the concentration of acetic acid in the water must be greater than that in the toluene. Thus, at the surface of the drop, we will experience an extra resistance which creates the discontinuity in our concentration gradients of acetic acid. In order to include this resistance into our overall mass transfer coefficient, we will have to consider the three controlling factors: the area of contact, the effective driving force, and the transfer coefficient, separately. We will perform analyses to develop relations for the coefficient of mass transfer inside the drop as well as outside the drop. Then, relate these coefficients to determine the overall mass transfer coefficient of our system. Dimensional Analysis The purpose of a dimensionless analysis is to predict the most important dimensionless parameters which are instrumental in modeling your situation. The situation that we will model is that of mass transfer between a liquid droplet flowing through another immiscible liquid phase. The important variables of our system are listed below in Table 1. Table 1: Important variables for Buckingham method Variable Drop Diameter Symbol Dimensions Fluid Density d m 3 ρ kg/m Fluid Viscosity μ kg/(m*t) Drop Velocity Fluid Diffusivity Mass Transfer Coefficient v m/s 2 DAB L /t K L/t Table 1: Important variables in our liquid-liquid extraction system. These terms are the parameters describing the geometry, velocity, and fluid properties of our system. Also the most important parameter, the mass transfer coefficient is also listed. Using the Buckingham method, we determine that there are six variables which span over three units, consequently our system will require three dimensionless 13 groups in order to model it. We define our core variables to be DAB , , and D , and can form our three dimensionless groups as the following a b D c kc (13a) 1 DAB d eD f v (13b) 2 DAB g h Di (13c) 3 DAB Writing 1 in terms of its dimensions, we arrive at a (14) b Length 2 Mass c Length 1 Length 3 Time time Length In order for 1 to be dimensionless, the exponents in the above equation must be a=-1, b=0, c=1 and our first dimensionless group must be (15) 1 Sc kc L DAB and is called the Sherwood number or the mass transfer Nusselt number. In a similar manner, we can solve the other two dimensionless groups and obtain Dv DAB (16) 2 (17) 3 Sc DAB Here, we have just introduced the Schmit number which is a ratio of the momentum diffusivity to the mass diffusivity, analogous to the Prandtl number for heat transfer. The dimensionless parameter 2 doesn’t add much in the modeling of our system, however if we divide 2 by 3 we obtain a familiar parameter known as the Reynolds number. 14 (18) Dv DAB Dv . Re DAB The final result of this dimensionless analysis suggests we will be able to correlate the mass transfer coefficient in our situation using (19) Sh function(Re, Sc) This is similar to that which was obtained in heat transfer, according to Thomas Salerno in his Convection Formal Report dated June 2006. (20) Nu function(Re, Pr) Terminal Velocity: Our theoretical treatment will begin in the same way we began our experimental treatment, by finishing the complete description of our situation. We are aware that we are extracting HAc from toluene into a spherical drop of water, however, we need a method to determine the speed that this drop is moving. To determine this, we again analyze Figure 3. FDrag FBouyancy FDrag Velocity FGravity Figure 3: Schematic of forces acting on drop. 15 It is important to note from Figure 3 that we have drawn the drag force as pushing the spherical drop up. This only happens when the velocity of the particle is down, which we know to be the case. Our math, however, will take this unknown direction of drag force into account. Then, we can re-apply Newton’s Law, which states that the sum of the forces is equal to the mass of the particle, multiplied by its acceleration. (1) M* C v v surrounding fluid Aprojected dv M M * agravity surrounding fluid agravity D dt drop 2 In this equation, M is the mass of the drop in kilograms, v is the velocity of the drop in meters per second, agravity is the acceleration due to gravity in meters per second squared, surrounding fluid and drop are the densities of the bulk surrounding fluid and of the drop respectively, both in kg per meter cubed, CD is the drag coefficient for a sphere, and Aprojected is the projected area of the sphere which is the area of a circle. Because of the dilute concentrations present in both the toluene and water phases, we can allow their pure density to represent the density of each phase. Thus, we can simplify Equation 1 to, (21) 2 dv agravity Wat Tol CD v v Tol * rdrop dt Wat 2* M We will assume that the drop reaches its terminal velocity immediately after entering the toluene. Therefore, our drop will be traveling at a constant terminal velocity vT, which is defined as, (22) 2 dv agravity Wat Tol CD v v Tol * rdrop 0 dt Wat 2* M In trying to solve Equation 22, we encounter an obstacle. The coefficient of drag is dependent on the Reynolds number, which depends on the velocity of drop. Also, we have different correlations between the coefficient of drag and the Reynolds number depending upon the value of the Reynolds number, which we 16 cannot calculate because we don’t know the velocity. These correlations were derived from an experiment and are given from Welty, Wicks, Wilson, and Rorrer to be: (23a) CD (23b) CD (23c) 24 for Re 2 Re 18.5 for 2 Re 500 Re.6 CD .44 for Re 500 . So in order to solve this equation, we have to guess a terminal velocity, choose the correct correlation for the coefficient of drag, insert this into Equation 22 and solve for the terminal velocity. Then, we calculate our actual Reynolds number and ensure we chose the right correlation, if not we have to select the correct correlation and solve again. Mass Transfer Coefficient Outside of Drop: We will now consider the transfer of HAc from the continuous phase of toluene to the surface of the spherical drop of water. As was done by Higbie, we can derive an expression for this outside mass transfer coefficient analytically. When the water drop descends down the column, through the toluene, a turbulent boundary layer will form around the sphere. We can analyze this boundary layer by using the typical boundary layer equations. Note the derivation of these equations is outside the scope of this report. For an abbreviated derivation, the reader should consult the Convection Experiment Formal Report written by Thomas Salerno. First, we perform a mass balance on the system and obtain continuity equation (24) vx v y 0 x y 17 Then, we can perform a momentum balance on the system and obtain the equation of motion, (25) vx vy 2vx vx vy x y y 2 Along with this momentum boundary layer, there will form a thermal boundary layer, which can be described by performing an energy balance on the system, (26) T T 2T vx vy 2 x y y Finally, we need an analogous differential equation for the mass transfer boundary layer which can be obtained by comparing the similarities in Equations 16 and 17 for momentum and thermal boundary layers. Each equation has the same form on the left hand side with the components of the velocities being multiplied by the state variables gradient in that direction. The right hand sides are also very similar with the derivative of the state variables gradient being multiplied by the particular equations’ diffusivity. Thus, we can write our mass transfer boundary layer equation as, (27) vx C A C A 2C A vy DAB x y y 2 Higbie, then, transformed Equations 24, 25, 26, and 27 into spherical coordinates. Assumptions were made that mass transfer occurs when differential volume elements of the continuous toluene phase comes into contact with the forward stagnation point in the drop. It diffuses the acetic acid into the drop as it travels around the drop. He formulated a conjecture following the work of Pigford, that the radial velocity at any point during the transfer is negligible, and that the tangential velocity is always equal to the terminal velocity of the falling drop. Finally, he used West’s postulation that the time of contact between the toluene phase and the spherical drop is simply calculated from, 18 (28) time d vT Where d is the diameter of the drop and vT is the drop’s terminal velocity. Note the mathematics in the current analysis to obtain Equation 29, although extensive, are rather straightforward. The derivation follows the normal calculation procedure for any boundary layer analysis. It will not be shown here because it is not the purpose of this report to solve straightforward equations, but, instead to show the techniques, theories, and assumptions used to arrive at these relations. If the reader would like to see the typical steps needed in boundary layer analysis, he or she should again consult the Convection Formal Report, written by Thomas Salerno After making these assumptions, the boundary layer equations can be solved to produce a correlation for the outside mass transfer coefficient in terms of the dimensionless variables we have previously defined, shown in Equation 29. Shoutside koutside 2 rdrop (29) koutside 1.13 DHAc 1/ 2 1.13Peoutside vT 2 rdrop 1.13 D HAc 1/ 2 1/ 2 vT1/ 2 DHAc 2 r 1/ 2 drop Mass Transfer Coefficient Inside of Drop The simplest approach to model the mass transfer inside the drop is to assume the drop is stagnant then apply and solve the ordinary differential equations presented above. However, this method will generally lead to mass transfer coefficients that are usually one or two order of magnitudes too low. This should be expected because there is motion between the two phases creating a convective mass transfer along with a diffusion mass transfer. 19 Because we currently don’t have an exact method to analyze this turbulence flow, it is impossible to solve this situation accurately. Therefore, developing a correlation for mass transfer inside the drop is much more difficult and will be discussed in detail in this section. According to Handlos and Baron, the circulation patterns in the drop can be represented as shown in Figure 4. Figure 4: Circulation patterns in the drop. In this schematic, Handlos and Baron combined the tangential motion of the drop with the (assumed) random radial motions due to vibrations in the evaluation of eddy diffusivities within the drop. The assumption of this circulation pattern is a valid one because experiments have been done comparing the velocity of falling solid spheres and falling liquid drops. That of spheres followed closely to its terminal velocity prediction (which assumes a rigid surface with only vertical velocity), however, that of the liquid drops shows large over predictions of the velocity. Also, observations of the drops in this experiment show some spinning of the drops which can be caused from this internal circulation in the drops. Finally, this circulation pattern can be given a more physical interpretation. Garner, Skelland, and Hale have shown that the transfer of solute from the continuous phase into the drop can cause such circulation patterns. 20 Handlos and Baron then replaced, for mathematical purposes, the streamlines in the drop by a system of tori shown in Figure 5. Figure 5: Drop with tori superimposed on the right. In this drop, particles are constantly being moved along streamlines due to the random radial motion. If we assume the circulation patterns are giving complete mixing inside the drop, then the probability that a particle is found between positions p and p dp is the ratio of the differential volume element at p to the total volume of the torus. Thus, we can form an equation for the position of any molecule as (30) P p dp 32 p dp d2 We can put this equation into a more useful form by using the substitution (31) r 4p d where d is the drop diameter. Combining Equations 28 and 29 gives (32) P r dr 2r dr . 21 We can develop a relation for the square of the displacement in our circular torus as, (33) z2 d2 2 r r . 16 If we run this experiment multiple times in our drop, the mean square displacement will simply be the expectation value of z 2 , which can be found via 1 (34) z 2 z 2 r P r dr 0 1 d2 d2 2 r r r dr 6r 2 8r 3 . 8 0 96 The average time required for this circulation was investigated and given by Kronig and Brink to be, (35) 16d i 1 , 3V o where V is the volume of the drop, i is the viscosity of the phase inside the drop, and o is the viscosity of the phase outside the drop. Proceeding to analyze the transfer process by eddy diffusion, we can represent our mass transfer through use of the Einstein equation, which combines the mean square displacement for a given time to the effective diffusivity. The result of this analysis of our situation yields, (36) 2 z2 dV 6r 8r 3 E (r ) . 4 2048 i 1 o 22 We can, then, multiply and divide by the molecular diffusivity, and obtain, after some manipulation, (37) E (r ) D Pe 6 r 2 8r 3 2048 where we have defined a new term, Pe , which is simply (38) Pe Pe i 1 o which uses a dimensionless parameter known as the Peclet number which is given by the multiplication of the Reynolds number and the Schmit number. Next, we apply the equation of continuity to our drop. For our drop this equation yields, in spherical terms, (39) c 16 1 c 2 E r . t d r r r where c is the concentration of solute within the drop. We can rewrite this equation into a more useful form by making the substitution r 1 y . We arrive at, after plugging in Equation 37, (40) 2048d 2 c 1 c 1 5 y 10 y 2 6 y 3 . 16 DPe t 1 y y y The boundary conditions for this equation are (41a) c co at t 0, 0 y 1 (41b) c 0 at y 0, t 0. 23 We can then solve Equation 40 and 41 by using the separations of variables technique. In this technique we assume that the concentration function we seek is made up of a function dependent only on time multiplied by a function dependent only on the position term. In mathematical terms we define, (42) c T (t )Y ( y ) We can then plug Equation 40 into Equation 38 and split this equation into a set of two ordinary differential equations: (43) dT 16 DPe T dt 2048d 2 (44) d dY 1 5 y 10 y 2 6 y 3 1 y Y 0 dy dy Where represents one of the many Eigen values. Accordingly the general solution of Equation 38 is (45) 16n DtPe c c0 AnYn exp 2 2048d 1 Where co is the initial concentration of the solute in the drop, An are the constants which need to be found from the boundary conditions, and Yn are the eigen functions corresponding to n . However, as is the case for most eigen-value problems, each successive Eigen value is larger than the first. In our present case, the second Eigen value is of sufficient magnitude that only the first term in Equation 45 is needed to model our system. Thus, we can write the ratio of the mass of solute in the drop at time t, to the amount present at time zero is, (46) M (t ) 16n DtPe 2 An 2 exp . 2 M (0) 2048d 1 24 By performing a material balance on our system, we can go back and obtain a relation for our mass transfer coefficient via another means. This yields, (47) d dc ki c * c 6 dt However, we can simplify this equation by noting the conditions we labeled in Equation 41, in which we arrive at, (48) d dc ki c * 6 dt From which, we can solve via the same method just described to yield a relation of the form, (49) M (t ) 6k t exp i . M (0) d Thus, we can equate Equations 46 and 49 to obtain the mass transfer coefficient expressed as a function of the first eigen value, (50) ki 161 DPe 6 2048 d 2 As long as we can find the first eigen value of Equation 44, we have a relation for the inside mass transfer coefficient. This can be solved using the Ritz method which finds an approximate eigen function via, j (51) Yn c j y j 1 With j=5 we are able to determine that the lowest eigen value is 1 2.88 . Thus, we can finalize Equation 50 into, 25 (52) ki .00375vT i 1 o We can rearrange this equation into the more general and useful form of, (53) Shi .00375Pe i 1 o which is the relation, developed by Handlos and Baron, that we will use in our experiment. Mass Transfer Coefficient Inside and Outside of Drop (Fudge Factor) Researchers Henschke and Pfenning studied the extraction from a falling drop into a continuous phase. They propose a similar argument for the theory of a falling drop which reinforces many of the ideas presented previously, introduced by Handlos and Baron. In fact, in the article, they agreed with the derivations and calculations made by Handlos and Baron. However, they did want to extend their theory a bit further. Because the model of Handlos and Baron was developed to be general for all liquid - liquid extraction systems, Henschke and Pfenning noticed that using this relation for liquid-liquid extraction brought with it a significant assumption, that the interface of the surface is ideal. They suggest that the mass-transfer induced turbulence will distort the internal eddies in a manner which is strongly dependent on the drop’s surface tension at its exterior. To account for this in Henschke and Pfenning’s own system, they only needed to add an extra term to Equation 53, (54) Shi .00375Pe CIP 1 i o 26 The constant, CIP , introduced in this equation is termed the instability constant and it characterizes the instabilities at the interface which is specific to the system. As it is difficult to foretell the surface tension in any particular system, they have not determined a method of predicting this constant. The exact compositions of each phase, the temperature, the pressure, the Reynolds number, etc. would have to be taken into account. Instead, they strongly recommended solving for it experimentally. Because they have determined the instability constant to be a substance-specific constant, we can calculate it after one measurement. Its value should stay constant throughout the experiment, or any similar extractions with the same materials at same temperature and pressure. Of course, our system is different than their system. In their development of Equation 54, they assumed the coefficient for mass transfer outside the drop is negligible, i.e., the inside coefficient is the controlling resistance. However, as our data will prove later, our system is such that the outside mass transfer is the controlling resistance. Similarly, Henschke and Pfenning did discuss this situation in their paper. They suggest, “if turbulence occurs simultaneously inside and outside the drop, the mass transfer resistance outside will be reduced by roughly the same factor as inside the drop. Thus we have to transform Equation 29 to Shoutside (55) koutside koutside 2 rdrop DHAc 1/ 2 1.13Peoutside 1.13 vT 2 rdrop CIP CIP DHAc 1/ 2 1/ 2 1.13 vT1/ 2 DHAc CIP 2 r 1/ 2 drop In their report, they suggested values from 100-2500 are reasonable for CIP, for turbulent systems. Overall Mass Transfer Coefficient: Now, we are prepared to determine the overall mass transfer coefficient governing our situation. This is accomplished by applying the two resistance theory. In this 27 theory, we need to determine separate relationships for mass transfer inside and outside the drop, which depend on their own driving force as if the other phase wasn’t present. These equations take the form, (56) N A kin y A,Wat y Ai (57) N A kout xAi xA,Tol Evidently, these two equations are equal due to the principle of conservation of mass, which states the moles leaving the outside of the drop has to equal the moles entering the inside of the drop, hence, we can write, (58) N A kin y A,Wat y Ai kout xAi xA,Tol . Note, that we have already developed relations to determine the mass transfer coefficient inside and outside the drop. The next step is to graphically relate the driving forces for both the inside and outside of the drop. Recall the driving force on the inside, is the concentration of solute on the inside less the concentration at the interface. Also, you must remember the driving force on the outside is the concentration at the interface less the bulk concentration in the continuous phase. The graphical relation is shown in Figure 6. y Ai y *A m' x Ai x AL y AG y Ai m" x*A x Ai Figure 6: Graphical representation of concentration difference driving force. 28 As exemplified in Figure 6, the driving force outside the drop can be modeled to a straight line with slope m . Similarly, we can fit the driving force inside the drop to a straight line with slope m . Consequently, we have defined the following two relations from our equilibrium graph. (59) m (60) m y Ai y *A x Ai x A,Tol y A,Wat y Ai x*A xAi Another important concept of note from Figure 6 and Equations 59 and 60, is that we are now able to link the driving force inside the drop to that outside the drop. This link was provided through the concentration of solute in each phase at the interface which is related by equilibrium. To create an equation for the overall mass transfer coefficient, we will utilize Figure 6, the driving force for the overall equation should be the combination of the driving forces for both inside and outside the drop. With this method, the concentration at the interface drops out and we are left with y A,Wat y *A . There may be some debate on what our driving force should be that of the inside or that of the outside. However, the choice of driving force doesn’t matter since we are algebraically defining the overall equation and not theoretically deriving it. One should realize that because of this flexibility, there will be two possibilities of the overall coefficient both of which will be different. However, both are acceptable, as long as they clearly stated which driving force coefficient they are using. For our experiments, we will base the overall coefficient off of the driving force on the inside of the drop, which we write as (61) N A Kin y A,Wat y*A Now, we can combine, substitute, and algebraically manipulate Equations 56 through 61, as follows, 29 N A K in y A,Wat y *A kin y A,Wat y Ai kout x Ai x A,Tol (62) y A,Wat y *A y A,Wat y *A add and subtract : y Ai y A,Wat y *A y A,Wat y Ai y Ai y *A substitute : m y A,Wat y *A y A,Wat y Ai m x Ai x A,Tol y Ai y*A x Ai x A,Tol substitute : N A K in y A,Wat y *A kin y A,Wat y Ai kout x Ai x A,Tol N A N A N A m K kin kout Thus we have derived a relation for the overall mass transfer coefficient to be, (63) 1 1 m . K kin kout 30 EQUIPMENT AND PROCEDURE: Part 1: Calculating Velocity and Mass Transfer Rate For the first part of the experiment, I inserted a drop of .01M NaOH in water, into a continuous stream of stagnant toluene which is at a previously unknown amount of acetic acid. The toluene phase was placed in a large 1-gallon graduated cylinder with markings on the side. This would allow the drop to fall vertically downward without any interruptions for at least .6 meters. In order to calculate the velocity of the drop, the experimenter utilizes the equally spaced markings on the side (.001775m per marking) of the graduated cylinder. At the bottom of the column, there is an outlet valve from which the toluene phase can be extracted to take a sample, and to facilitate the empting of the column when the experiment is done. These features can be seen in Figure 7. Vo lu metric Flo w: mL per Minute Vo lu metric Flo w: mL per Hour Figure 7: Schematic of graduate cylinder used to hold toluene phase. In order to insert the drops at the same height and location in the column, a cut syringe which hung from a circular clamp just above the graduated cylinder was used. A cut syringe was preferred for this experiment as it was constructed with a 31 plastic tip. By simply cutting the plastic tip on the syringe to a required size, the diameter of the drop leaving the syringe was at a constant diameter throughout each portion of the experiment. Additionally, the syringe was fixed to a plunger which laid on an syringe pump. To assist in the regulation of the amount of liquid being pushed out of the plunger per unit of time from the syringe the syringe pump Model 341 from Sage Instruments, a division of Orion Research Inc., was required. This automated pump regulated the rate of compression on the plunger to ensure the same pressure would be used to expel a drop from the syringe which would in turn ensure constant velocity and diameter of the drop leaving the syringe. The controller on this unit allowed for a number of settings ranging from a fast to slow output. Thus, allowing all three members of my group to accurately record the time for the drops to fall a certain distance. Finally, the plunger was connected to a sample pot filled with the .01M NaOH solution, which was closed to the environment, to guarantee proper loading of this sample without any evaporation or air pockets which could distort either the sample itself or the drop size. The features for this part of the apparatus are shown in Figure 8. Vo lu metric Flo w: mL per Minute Vo lu metric Flo w: mL per Hour Syringe Pump Model 341 Sage Instruments Figure 8: Schematic of syringe, plunger, sample pot, and syringe pump Model 341. Having described the equipment to be used during the procedure, I will begin to explain the course of action for this part of the lab. The first variable that must be 32 measured is the volume of the drop at the current syringe size. To correctly manage the volume of the drop, a sample was loaded into the plunger which was connected to the automatic dispenser, and set on .54mL/min. A high setting is practical at this point, as we had time restraints and a certain number of drops needed to be calculated. We then proceeded to place 50 or 60 drops into a 10mL graduated cylinder and recorded the volume of these drops. Then, we divided by the number of drops which allowed us to solve for our volume per drop. The next variable of interest is the drop’s terminal velocity. To make this calculation easier, we made certain that the toluene phase started exactly at the zero line on the graduated cylinder. We used a ruler to calculate the distance it takes for 600 marks to be passed, namely, .213 meters. Once the drop enters the toluene, at this zero line, we will start the first stopwatch. As it passed the 600 line, the first stopwatch was stopped and the second stopwatch started. Sequentially, when the drop reached the 1200 line, the second stopwatch was stopped and the third stopwatch started. Till finally, when the drop reaches the 1800 line the third stopwatch is stopped. All of these time recordings measure the time it takes for this drop to pass .213 meters, but in different locations of the tube. To find the terminal velocity of the drop, these values can be averaged. The experimenter should also note the average time in each section of the cylinder in order to reveal if the particle is at a terminal velocity or if its velocity is changing throughout the cylinder. Because of the necessity of accuracy, it is recommended to take 10 trials of this procedure. For both the determination of the drop’s terminal velocity and the determination of the mass transfer rate, it is recommended to set the syringe pump at .12mL/hr, thus allowing time to take measurements and record them before the next drop falls. The next part of the experiment requires the experimenter to ascertain the time it takes this particle to turn clear. This method is similar to that used to find the drop’s terminal velocity. Simultaneously, all three experimenters will start their stopwatches when the drop enters the toluene phase. Of special note, is that the particle must be falling when it reaches the toluene phase, if it is still hanging from the syringe but not falling, as it sits in the toluene phase it will be 33 transferring mass of the acetic acid before you can begin your timed experiment. One must ensure the syringe is far enough above the toluene phase and that the drop is completely in free fall before it enters the stagnant continuous phase. The drop will be monitored by the three evaluators as it travels downward in the column. Each of the three participants will then bring to a halt their stopwatches at the moment they deem the drop has turned completely clear. Again, the experimenter should note that the pink will disappear from the bottom of the drop first but still remain in the top of the drop, do not stop the stopwatches until the drop has completely turned clear. It is recommended to execute 10 trials of this part of the procedure to gain proficiency at documenting a clear drop versus a slightly pink one. After each trial record the time, as this will be used to calculate the mass transfer rate. Part 2: Titration For the second part of this lab, standard titration equipment was needed. This included a 5 ml pipette , a 1 ml pipette, two 100mL titrating graduated cylinders with control valves at the bottom secured to a rotating stand, a 250 ml beaker, a 250 ml separatory funnel, and a magnetic stirrer. These materials will be arranged as seen in Figure 9 Figure 9: Schematic of titration equipment. 34 The concentration of acetic acid present in our toluene phase is the first variable we want to calculate in this stage of the lab.. In order to do this, place a magnetic stirrer into a 250 ml beaker. Then, place 5ml of your toluene phase solution from your column into this beaker using the 5ml pipette. Next, place 3 drops of phenolphthalein solution into the beaker. Place the beaker on the electronic stirrer and mix the solution. After that, add pure NaOH from one of your titrating cylinders slowly into your solution until you achieve a light pink color. Record the amount of NaOH needed for this. Rotate your HCl titrating cylinder to be over your solution. Add HCl very slowly into your mixture to reach a fading pink endpoint, the point where your solution is only slightly pink and about to turn clear. Record the amount of HCl needed. From this data you should be able to calculate the amount of acetic acid present in your toluene phase from the following calculation, mmole HAc mL NaOH mL HCl (64) CHAc , Tol .1 mmole mL mmole HAc 5 mL sample Next, the experimenter needs to calculate the distribution coefficient of acetic acid in water and toluene. To accomplish this, we need to mix a given amount of water, toluene, and acetic acid, and measure the concentration of acetic acid present in each of the water and toluene phase after mixing. However, we should note that this value is concentration dependent. Thus, we must calculate the amount relative proportions of each component that we need to add such that the concentration of acetic acid present in the toluene phase is equal to that we have found in our column. To do this calculation, we first assume a distribution coefficient, usually a value of 10 is a good estimate. Then, we approximate the amount of acetic acid we would need to add in order to obtain the same concentration of acetic acid in our toluene phase that we recorded from our column. This calculation procedure is as follows, 35 mmole mL mmole mmole HAc in Wat 50mL Wat *10*.8 mL mmole HAc mmole HAc in Tol mmole HAc in Wat mmole HAc in Tol 50 mL Tol * .8 (65) mL HAc mmole HAc * mole 60.05 g 1mL * * 1000mmole mole 1.05 g After this is completed, mix equal amounts of pure water and pure toluene (in the amounts used for the calculation in Equation 65 and add the appropriate amount of acetic acid, the amount calculated from Equation 65. Mix this solution for approximately 30 minutes to ensure there is good intermingling between these two immiscible phases which will guarantee the acetic acid will separate according to equilibrium. Next, place the mixture into a separatory funnel and let it settle for about a minute. Take a 5ml pipette and extract the toluene (top) phase from the funnel itself. Continue to titrate with this via the same method described previously. The concentration of acetic acid in the toluene phase can be calculated through the use of Equation 64. Remove a small amount from the bottom of the separatory funnel and place into a small jar. Use a 1ml marked pipette to transfer .5ml of this into a beaker. Add water from a squirt bottle around the sides to make sure the entire .5ml is in solution. Then, add three drops of phenolphthalein, and titrate with HCl until the solution reaches its faded pink endpoint. Using similar equations to Equation 64, the experimenter should be able to calculate the amount of acetic acid present in the water phase via, mmole HAc mL NaOH (66) CHAc , Wat .1 mmole mL mmole HAc .5 mL sample With this data, the experimenter can use Equation 7, and find the distribution coefficient as, 36 (7) C m HAc ,Wat C HAc ,Tol . This will complete the calculations and experimental procedure for this lab. The experimenter would now follow the theory section of this report to calculate all of the parameters needed to satisfy the objectives of this experiment. 37 RESULTS AND DISCUSSIONS: Terminal Velocity: In analyzing our data, our first step was to determine the terminal velocity of our falling drop. As described previously in our theory section, we have three forces acting on our particle: Gravity, which pulls the molecule down; Buoyancy which pushes the molecule up; and Drag, which acts to retard the molecules velocity, in our case the Drag will always be pushing up since our drop is always traveling downward. These forces will act on the particle via Newton’s Second law which allows us to model the drops velocity as, (21) 2 dv agravity Wat Tol CD v v Wat * rdrop dt Wat 2* M To determine this value theoretically, we simply have to solve Equation 21 for v, while setting dv 0 , which is the definition of terminal velocity. This maybe a dt bit tedious because the correct correlation for CD depends on the value of the terminal velocity as given by Equations 23a,b, and c. However, a simple guess and check procedure was used and worked satisfactory in obtaining the required value. To determine the experimental value, we has to assume that the particle reaches terminal velocity almost immediately after entering the toluene phase. Thus, we released a drop in the toluene phase, recorded the time it needed to vertically travel down the column’s first .213 meter section, then recorded the time for the drop to travel down the column’s second .213 meters, and finally the time needed for it travel the column’s third .213 meters. The averages for these times are shown in Table 2. 38 Table 2: Average Times (sec) for Drop to Fall .213m Increments Division of Column Size of Drop: First .213m Second .213m Third .213m Small 2.19 2.33 2.24 Medium 1.98 2.20 2.05 Large 1.68 1.88 1.82 Table 2: Average times recorded for drop to fall .213m in the column. From this table, it is evident that the first .213m generally traveled in the shortest time. However, its values are in close approximation to the other average times. This validates our assumption about the molecule reaching its terminal velocity immediately and is acceptable to give us the same results we would have obtained if we didn’t make this assumption. Subsequently, we can now calculate the experimental terminal velocity by dividing the average time it took each drop to travel the specified .213 meters. The results of this calculation as well as the results of theoretical calculations are shown together in Figure 10. Terminal Velocity of Drop as a Function of Drop Diameter 0.18 y = 1.8303x - 0.0327 R2 = 0.9754 0.16 Terminal Velocity (m/s) 0.14 0.12 0.1 Experimental Velocity Predicted Velocity Linear (Predicted Velocity) 0.08 0.06 0.04 0.02 0 0.07 0.075 0.08 0.085 0.09 0.095 0.1 0.105 0.11 Square Root of Drop Diameter (m^.5) Figure 10: Terminal velocity of the drop (Experimental and Predicted) as a function of drop diameter 39 It should be noted from this graph that as the diameter of the particle rises, the terminal velocity of the particle rises. The reader should also observe that at all diameters our theoretical predictions are consistently overestimating the velocity by 21%. The data for this is shown in Table 3. Table 3: Measured and Predicted Terminal Velocities of Drop Diameter (m) Measured (m/s) Error Predicted (m/s) Error Experimental Error 0.00346 0.095 5.0% 0.117 3.1% -19.5% 0.00398 0.103 6.4% 0.134 3.2% -23.1% 0.00516 0.119 6.1% 0.152 3.5% -22.0% Table 3: Measured and Predicted Terminal Velocities of Drop The result of the terminal velocity increasing as the diameter increases is logical from a simple thought analysis prospective. As was previously mentioned, the drop has three forces acting on it, a force from gravity always pushing the molecule down, a buoyancy force from displacing the toluene which always pushes the molecule up the column, and a drag force which always acts opposite the molecule’s velocity. We know that the force of gravity is driven by the weight of the particle, which is mostly water and that the force of buoyancy is driven by the weight of the continuous phase which it has displaced, which is mostly toluene. Since the drop is the same size, we can compare these forces by comparing the densities of water to toluene. In view of the fact that the water is denser than toluene the force of gravity will be greater and the particle will fall. Therefore, if we increase the diameter of the particle, we also increase its volume and since the densities remain constant, the force due to gravity has to grow greater in proportion to the force due to buoyancy. Clearly, this implies that as the diameter of the particle grows, the greater the force pulling the particle down is, thus. the velocity of the particle must increase, which is exactly the results we see here. (Note we have neglected the drag force in this analysis, but this force is dependent mainly on the velocity of the particle and less on its size for the range of sizes with which we have experimented. For that reason, its effect on causing the magnitude of the velocity change is substantial, but its effect on causing the velocity to either increase or decrease is negligible for our purposes.) 40 To explain the reason for the substantial over prediction of the terminal velocity of our experiments, we need to consider the assumptions being made. In order to develop our theoretical equation for describing the velocity of the falling particle, we had to assume that the particle remains a perfect rigid sphere. However, as the analysis of Handlos and Baron have proven, the molecule is continuously oscillating the material on the inside and also changing shape and oscillating the material at the surface, due to the mass transfer and the lack of rigidity of liquids. Thus, in our theoretical model, Equation 21, our assumptions predict a straight vertical drop of our particle. Where, in reality, we have observed our particle to fall in more of a helix pattern. This helix flow path is caused by the internal circulations of the particle and has the effect of increasing the drag coefficient of the particle as it falls. This result was proven by Bond and Newton. Hence, the increased drag coefficient on our particle will cause the terminal velocity of our particle to be reduced as compared to the value we have predicted. As a result, the experimentally measured value of the terminal velocity is a more accurate answer and will be used as the velocity in all of our calculations in this experiment. The argument has been brought up that the experimentally measured value is also off because the helix path of the particle is causing the actual distance the particle is traveling to be more than the measure .213m of the column. However, we take the classic physics assumptions that the velocity in the vertical direction is independent of the velocity in the horizontal direction, then as long as the forces on the particle are always acting vertically; our experimental measurement will still be accurate. Concentration of Acetic Acid in Toluene: The next step of my analysis was to calculate the amount of acetic acid that was present in my toluene phase in the column. This was accomplished through titration. I added 5 ml of my toluene phase in a beaker and than added an excess of NaOH to ensure that all of the acetic acid had reacted. This was an important step in the process as NaOH does not mix well with the toluene phase. Then, I back titrated 41 with HCl and calculated the amount of acetic acid from subtracting the amount of NaOH needed, from the amount of HCl needed to reach the phenolphthalein endpoint. .776 mol/L of acetic acid in my toluene was my end result. This molarity was the outcome of my adjusting the unknown molarity of the original toluene phase present in the column, such that the large drop of NaOH will turn clear roughly ¾’s of the way down the column. Thus, there is no predicted value this concentration should match. However, I did compare this with other values recorded from other students who performed the same experiment and it was in the same ballpark as their answers, in consequence, I have confidence this value is accurate. Due to the time restraint in running this lab, I was only able to titrate this mixture twice, therefore my error is simply the standard deviation of two points. This value is probably not as accurate as I would like but is all I can work with, only given two – three hour labs. Thus, the error is .037 mol / L . This data is shown in Table 4. Table 4: Molarity of Toluene Phase Current Expriment: Concentration of HAc 1 Concentration of HAc 2 Average Conc HAc Std Dev Conc HAc Concentration of Hac: 0.75 0.802 0.776 0.037 mol/L mol/L mol/L mol/L Previous Experiments: Concentration of HAc 1 Concentration of HAc 2 0.803 mol/L 0.813 mol/L Table 4: Concentration of HAc in the toluene phase. Distribution Coefficient: The next step of the calculations was to find out the distribution coefficient for our system. In other words, I need to determine how the acetic acid will distribute itself when it is in equilibrium with water and toluene. 42 In order to determine this, I mixed 50 ml of both water and toluene with 27 ml of acetic acid and stirred the mixture until it was uniform (about 30 minutes) and allowed it to settle. I proceeded to titrate each phase of the two phase mixture to find the concentration of acetic acid in each phase. From this, I was able to calculate the distribution coefficient based of the concentration of acetic acid in each phase following Equation 7. (7) C m HAc ,Wat C HAc ,Tol The results of this calculation are shown in Table 5. Table 5: Experimental Distribution Coefficient Phase: Toluene Water Distribution Coeff - m Assumed Error - m Concentration of Hac: 0.803 mol/L 8.54 mol/L 10.635 1.01 Table 5: Experimentally determined distribution coefficient. Because of the time constraint placed on my lab, I only had sufficient time to run this portion of the experiment once. As a consequence, I was not able to calculate my values through an average, nor was I able to find my experimental error through standard deviation. However, examining this process in detail, I am able to perceive that the only probable place of error could occur during my titration calculations. Thus, it is a good assumption that my titrating ability is fairly consistent and the error associated with each titration is equal in percent error obtained during my previous titration calculation (4.8%). The error associated with the distribution coefficient should be the sum of each of the errors of the two titrations, and it is this value that appears in Table 5. In an attempt to compare this with theory, I again encountered a problem. There is currently no accurate way to predict the distribution coefficient of my system at its exact concentration. However, once again, I can use previous experimental 43 results of this calculation for my theoretical prediction. Although this time, the experiments were controlled accurately and recorded in literature as an average of multiple runs, thus, my confidence in this predicted value will be higher than it was for the predicted value of the molarity of the toluene phase. According to Fuse and Iguchi, for my system of 8.0 mole percent acetic acid in the toluene phase, the distribution ratio should be 2.32 .132 mole percent in water phase / mole percent in toluene phase. Through some data, I can convert their mole percent distribution coefficient can be converted to a value of 9.0 .512 . This data can be combined in a more user friendly way by use of Table 6. Table 6: Experimental and Predicted Distribution Coefficient Value Basis Measured m 10.635 concentration Error in Measured m 9.5% concentration Predicted m 2.32 mole Predicted m 9 concentration Error in Pred m 5.7% concentration Experimental Error 18.2% Table 6: Experimental and Predicted distribution coefficients. With the help of this table, we can see that our measured distribution coefficient is close to the predicted value but still substantially far from it with 18% error between the two values. However, this error is acceptable, because as stated before, the data used to make our prediction was based on past experiments. Thus, this isn’t an absolute calculated value, just another group of researchers’ experimental prediction for its value. The fact that our two distribution coefficients doesn’t match, doesn’t imply any failure in this experiment, but the fact that the two values are close in approximation afford me confidence in my results. Overall Mass Transfer Rate: 44 To satisfy our next objective, we need to calculate the mass transfer rate of acetic acid entering the drop for each diameter. To measure this value experimentally is relatively straightforward. We have previously calculated the volume of each drop that we add to the system, and we have set the concentration of NaOH in the drop equal to .01M. Thus, we can calculate the amount of NaOH present in each drop by multiplying the two afore mentioned quantities. This value will also be the amount of HAc needed to enter the drop to completely neutralize the NaOH, because this reaction has a one to one ratio of these components as given from Equation 3. (3) CH 3CH 2COOH (aq) NaOH (aq) H 2O(l ) CH 3CH 2COO Na (aq ) Next, we can measure the time needed for the drop to be completely neutralized, by calculating the time needed for the previously calculated amount of acetic acid to enter the drop. Dividing these two values will give the molar rate of transfer as evidenced by Equation 6. (6) n N HAc HAc ,extracted time The possibility of error can penetrate this procedure in one of two areas. First, the error in calculating the volume is derived from the error in reading the volume of a certain number of drops, in a 10 ml graduated cylinder, which will be .1 mL . The second area of possible error will be in recording the time needed for complete neutralization. This value can be calculated from the standard deviation of all of the measurements taken to record this time and is ±.22 seconds. It should be noted that this error is probably much lower than the actual error, because each of the participants have a different perspective of the exact point the drop turned clear. This data is compiled into Figure 11. 45 Molar Transfer Rate as a function of Drop Diameter 15 Molar Transfer Rate (mol per second *10^-8) 14 13 y = 4058.2x - 8.0895 R2 = 0.9984 12 11 Molar Transfer Rate Linear (Molar Transfer Rate) 10 9 8 7 6 5 0.003 0.0035 0.004 0.0045 0.005 0.0055 Diameter of Drop (m) Figure 11: Molar Transfer Rate as a function of drop diameter. The most important aspect of this graph is the functionality of the molar transfer rate with drop diameter. It seems the molar transfer rate increases almost linearly as the diameter increases. It is impossible for me to be fully confident that the relationship is purely linear because of the few number of data points, however the strong correlation coefficient does suggest it is most likely linear. The result of the molar transfer rate increasing with increasing diameter is hard to prove logically at this point. We know that the molar transfer rate, according to Newton’s Laws, should be a function of how the surface area depends on diameter and how the overall mass transfer coefficient depends on diameter. As for the latter, we can be sure the surface area increases with increasing diameter simply from the formula for surface area. However, we have yet to determine the relationship for mass transfer coefficient. Thus, we will return to this topic of intellectually proving or disproving this result later in the report. Overall mass transfer coefficient: 46 To determine the overall mass transfer coefficient experimentally, is again, a relatively straightforward task. We have previously calculated the molar transfer rate. From Equation 11 all we need to calculate the overall mass transfer coefficient (based on the inside mass transfer) is the equilibrium concentration of acetic aid at the surface in the water phase. (11) K N HAc m * CHAc,Tol * A The C* can be easily calculated from the definition of the distribution coefficient. The distribution coefficient, that we have previously calculated to be 10.64, is the concentration of acetic acid in water divided by the concentration in acetic acid in toluene which would be achieved when the phases are in equilibrium. Since, we have already calculated the concentration of acetic acid in the toluene, the concentration of acetic acid in the water at the surface of the drop (which, from our assumptions, must be in equilibrium with the toluene) can easily be calculated from (7) C m HAc ,Wat C HAc ,Tol . The error associate with this calculation is easily calculated from the error associated with the distribution coefficient (previously found) and the error associated with the calculation of the concentration of acetic acid in the toluene (previously calculated). To determine the theoretical value for the overall mass transfer coefficient is much more complicated. It involves using many assumptions and calculations introduced by Handlos and Baron, which were explained in detail in the theory section of this report. The equations derived from this rigorous analysis are 47 Shoutside koutside 2 rdrop (29) koutside 1.13 DHAc 1.13Pe 1/ 2 outside vT 2 rdrop 1.13 D HAc 1/ 2 1/ 2 vT1/ 2 DHAc 2 r 1/ 2 drop (53) Shi .00375Pe i 1 o The error introduced by these calculations can be easily seen from the variables involved in these calculations, the error in velocity (mainly associated with the standard deviation of the multiple time recordings), the error in recorded diffusivity (can be assumed to be low), the error in recorded viscosities (can be assumed to match the same percentage as recorded density), the error in the radius (derived from the error in reading the volume of 50 drops). To satisfy our objective of determining how the overall mass transfer coefficient depends on diameter, I have graphed the data of in Figure 12. 48 Overall Mass Transfer Coefficient as a Function of Drop Diameter 295 Overall Mass Transfer Coefficient (10^-7 m/s)) 245 195 K Exp K Pred Linear (K Pred) 145 95 45 -5 0.003 0.0035 0.004 0.0045 0.005 0.0055 0.006 Diameter of Drop (m) Figure 12: Overall Mass Transfer Coefficient as a function of drop diameter The most important thing to note from Figure 12 is that the overall mass transfer coefficient drops as diameter increases. However, it is also key to notice the significant error obtained in our prediction of the overall mass transfer coefficient using Handlos and Baron’s equations. The overall mass transfer coefficient is a proportionality factor added to the rate equation. Its purpose is to measure the effectiveness of the surface area at achieving mass transfer. In other words, it measures the resistance of the drop at transferring the acetic acid across its surface. The result of decreasing overall mass transfer coefficient with increasing diameter is realistic from a logical point of analysis. If we view this system from outside of the drop’s perspective, as we have previously discussed in the theory section of this report, there is convective mass transfer (at turbulent levels) due to the drop moving through the continuous phase at its terminal velocity. However, as was mentioned in the theory of Handlos and Baron, there is also a considerable amount of turbulence induced by the transfer of mass occurring at the interface. This mass transfer acted to cause 49 turbulence due to radial motion of particles inside the drop which formed large eddies currents, which aided mass transfer. These eddies distortions are based off of two characteristics of the system. First, the rate of mass transfer at particular points along the surface, and secondly, the volume of the particle because as represented from Figure 5, after the particles are induced to move radially by mass transfer they must travel a path around half the volume of the molecule until they are forced back to the surface. Therefore as the diameter of the molecule increases, so does its volume, and the path that these molecules must traverse also becomes larger. But since the surface area has grown less, as compared to the volume, the added velocity of these particles is not enough to overcome the extra distance and consequently, the eddie currents must move slower. These slower oscillations should cause less of a mass transfer induced turbulence and result in the area to be less effective at mass transfer as the diameter increases. However, if we analyze this from inside the drop’s perspective, the result of the overall mass transfer coefficient decreasing with increasing diameter can seem inaccurate. Because the volume of the drop is increasing faster than the corresponding surface area, we see that the ratio of molecules further away from the acetic acid is growing relative to the molecules in equilibrium with the acetic acid at the drops surface. Thus, we have a higher proportion of molecules that will have reacted with the NaOH and have zero concentration of acetic acid. Despite the slower eddies, the increase in the molecules with no acetic acid should have an overall effect of increasing the average driving force among all of the molecules present. Therefore, the effective driving force among all molecules would have increased and we would expect the overall mass transfer coefficient to increase with increasing drop diameter. As a result, we now have a quandary. Why is the first explanation correct and the second explanation wrong? This question will be answered later in my report. The result of our model over predicting the mass transfer coefficient is not surprising. As was discussed in the Fudge Factor section of the Theory in this report, many researches have felt that the model produced by Handlos and Baron 50 was very accurate in its analysis; however, they assumed ideality in their mass transfer system. This is because Handlos and Baron, developed their model to be general and applicable for all liquid - liquid extraction systems. On the other hand, as pointed out by Henschke and Pfenning each liquid – liquid extraction system comes with certain non negligible instabilities at the surface such as surface tension and coalescence, which will cause the mass transfer coefficients to be less. They suggest only the addition of a single constant to adjust for this, though, they do point out this constant, although relatively consistent for every similar system, must be measured experimentally. With this understanding, I decided to model my system using their adjusted equations, (54) Shi .00375Pe CIP 1 i o Shoutside (55) koutside koutside 2 rdrop DHAc 1/ 2 1.13Peoutside 1.13 vT 2 rdrop CIP CIP DHAc 1/ 2 1.13 vT1/ 2 DHAc CIP 2 r 1/ 2 1/ 2 . drop As you can see from Equations 54 and 55, the instability constant can be easily factored out to give K pred CIP K exp . We are able to find this value by graphing the originally predicted overall mass transfer coefficients versus the experimentally determined mass transfer coefficients, in which case the slope of the graph should be CIP. This can be seen in Figure 13. 51 Predicting the Instability Constant 224 K pred orig (10^-7 m/s) 222 220 y = 113.28x + 2.0928 R2 = 0.9953 218 216 214 212 1.86 1.87 1.88 1.89 1.9 1.91 1.92 1.93 1.94 1.95 K exp (10^-7 m/s) Figure 13: Graph of Kpred versus Kexp to determine CIP A statistical regression of this data in Excel provided me with CIP 113.28 7.28 . After adding this instability constant, my predicted and experimental results showed good conformity as evidenced by Figure 14. 52 1.96 Overall Mass Transfer Coefficient as a Function of Drop Diameter Experimental versus Predicted with Fudge Factor 2.5 Overall Mass Transfer Coefficient (10^-7 m/s)) 2.4 2.3 2.2 2.1 K Exp K Pred FF Linear (K Pred FF) 2 1.9 1.8 1.7 1.6 1.5 0.003 0.0035 0.004 0.0045 0.005 0.0055 Diameter of Drop (m) Figure 14: Fudge Factor predicted and experimental overall mass transfer coefficient as a function of drop diameter The most significant aspect of Figure 14 is that with the addition of a simple instability constant, the predicted values are within 1.5% error of the experimental values and follow the same functionality with diameter, each averaging a 2% drop in the value of the overall mass transfer coefficient with a 1mm increase in diameter. Individual mass transfer coefficients: We now return to the question of what is causing the overall mass transfer coefficient to decrease with increasing diameter. The answer lies in the functionality of the individual mass transfer coefficients with increasing diameter. Analysis of the individual mass transfer coefficient reveals that they follow different trends with diameter. As shown in Figure 15, the mass transfer coefficient outside the drop decreases with increasing diameter, where as the mass transfer coefficient inside the drop increases with increasing diameter. 53 Individual Mass Transfer Coefficient as a Function of Drop Diameter 3 Individual Mass Transfer Coefficient (10^-4 m/s)) 2.8 2.6 2.4 2.2 K inside drop K outside drop Linear (K outside drop) Linear (K inside drop) 2 1.8 1.6 1.4 1.2 1 0.003 0.0035 0.004 0.0045 0.005 0.0055 Diameter of Drop (m) Figure15: Individual mass transfer coefficients as a function of drop diameter. The most vital aspect the reader should take away from Figure 15, is that the outside mass transfer coefficient follows the same trend as the overall mass transfer coefficient, where as the inside mass transfer coefficient increases with increasing diameter. Therefore our explanation from outside of the drop’s perspective as to why the mass transfer coefficient decreases with increasing diameter is true and this is reflected by the trend of the outside mass transfer coefficient. Also, our explanation from inside of the drop’s perspective as to why the mass transfer coefficient increases with increasing diameter is true and is reflected by the trend of the inside mass transfer coefficient. It is with confidence in our thorough analysis that we can conclude that the overall mass transfer coefficient follows the trend of decreasing with increasing diameter because the outside mass transfer coefficient is the controlling resistance 54 in our system. This is because its value of resistance is larger and is combined with the distribution coefficient which represents the discontinuity resistance of the acetic acid concentration at the surface. Revisiting the Overall Mass Transfer Rate: Now, let us revisit the trend we observed in the overall mass transfer rate as we have accumulated pertinent data and information to process this information. Recall from earlier in this report, we showed that the mass transfer rate increases with increasing diameter. This result agrees with what we would expect if we performed a rational analysis of this situation. Starting form Equation 9 (9) N HAc K * A * C * CHAc ,Wat We can see that the overall mass transfer rate depends on the overall mass transfer coefficient, the surface area, and the driving force. If we change the diameter of our particle, there should be no effect on our driving force, although the total number of moles of acetic acid transferred would be different, the driving force which is based on the concentration would stay the same. This means the functionality of N with diameter must be based on only K and A. The overall mass transfer coefficient we have previously found to fall with increasing diameter, while the surface area is easily proven to rise with increasing diameter. Hence, it depends on which of these terms rises faster relative to the other, which will control the direction the overall mass transfer rate will follow. There is no definite theory that one of these terms always has to grow faster than the other, however, since we have already proven that the mass transfer rate increases with increasing diameter, we should expect that the surface area is growing relatively faster, and is the controlling factor in determining N. This can be proved through the use of Table 7 55 Table 7: Percent Growth of S.A. and K Surface Area (sq m) Percent Change K overall (m/s) Percent Change Diameter 0.00346 3.76E-05 Initial 1.95E-07 Initial 0.00398 4.98E-05 32.4% 1.92E-07 -1.5% 0.00516 8.37E-05 68.2% 1.87E-07 -2.9% Table 7: Percentage Growth of the drop’s surface area and overall mass transfer coefficient. This table shows that the percentage increase in surface area as diameter increases is much greater than the percentage decrease in the overall mass transfer coefficient. Accordingly, our observation of the mass transfer rate increasing with increasing diameter is logical in conclusion. 56 CONCLUSIONS: The purpose of this experiment was to provide a complete description of the extraction of acetic acid from toluene into water. This unit operation was studied through micro extraction, measuring the molar transfer rate, overall mass transfer coefficient, and the equilibrium driving force of the transfer of acetic acid in a stagnant continuous toluene phase into a single droplet of water (which also contained NaOH and phenolphthalein). In this experiment we discovered the equilibrium distribution coefficient for acetic acid in water and toluene is 10.63. This result was 18% higher than the value obtained by Fuse and Iguchi, in their experiments. The percent error obtained in my experiment was found to be 9.5%. The percent error in Fuse and Iguchi’s experiment was estimated to be 5.7% based on the scatter in their data. However, it is not unreasonable to assume that there may be other errors present which is not represented by the scatter in the data. Thus, it is not unreasonable to believe their total experimental error, including the scatter and any uncertainty in the values of the physical parameters they must have used in their calculations, is also close to 9.5% in this range of concentration of acetic acid in toluene. Therefore, the two values for the distribution coefficient are in agreement. Table 6 summarizes the data from this portion of the experiment. As a result, we can conclude from this experimental analysis that the distribution coefficient for an industrial liquid-liquid extraction system involving these components is 10.63. This value can be used in making the mass transfer calculations around this unit operation to ensure we set the correct parameters to achieve the desired separation. Of course, in order to use this value, the engineer will have to assume that the system is able to obtain equilibrium at the interface of the two immiscible liquids. The next objective of this experiment was to determine how the terminal velocity of the falling drop depends on diameter. It was easy to predict, before performing this experiment, that as the diameter of the particle increased, the terminal velocity would also increase. This was easily accomplished by looking at the 57 forces acting on the falling drop and using Newton’s second law to relate the sum of the forces. This prediction should be simple for any certified engineer. However, it wasn’t the trend that was the most important part of this objective, instead it was the ability to determine an experimental correlation and see if it differs from that predicted by theory. As can be seen from Figure 10 and Table 3 the predicted trend of increasing terminal velocity with increasing diameter is proven. However more importantly, notice the large amount of error between the predicted values and the experimentally measured values. This 21% average percent error reveals that this system does not travel in the ideal fashion predicted by our theoretical equations. This proves that the drag coefficient of our drop is significantly large than that which would be expected by a solid rigid sphere. Allowing us to conclude that our drop doesn’t travel down the column as a sphere, but instead will distort itself. Also, this distortion will cause oscillations or eddies in the drop. These eddies will act to slow down the particle having it result in much lower velocities as we see here. But more importantly, the major significance of proving the larger error in the terminal velocity prediction is the proof of the formation of these eddies. As described by Handlos and Baron and outlined in the Theory section of this report, the formation of eddies is key to model the turbulence involved in the mass transfer in this system. Additionally, this data provides a correlation for engineers to predict how the oscillating particle’s terminal velocity truly depends on drop diameter. This functionality will be useful in the engineer’s design of an extraction column. The next objective was to determine how the overall mass transfer coefficient depends on diameter. To completely answer this question, it was easier to see how the individual mass transfer coefficients depended on diameter. From Figure 15, the reader should observe that the outside mass transfer coefficient decreases as the diameter increases. It was my conclusion that the increase in the volume to surface area ratio caused the internal oscillations (eddies) to become relatively slower. Hence less turbulence induced mass transfer was occurring and causing 58 the additional surface area to become less effective. Also from Figure 15, the reader should take note that the inside mass transfer coefficient increased with increasing diameter. This led me to the conclusions that the increase in volume, allowed for a higher percentage of molecules to be present with zero concentration of acetic acid (i.e. away from the surface), as compared with those molecules in equilibrium with the acetic acid (i.e. at the surface). Thus, the overall average driving force will be higher and make the surface area more effective for mass transfer. By looking at Figure 12, the evidence reveals that the overall mass transfer coefficient was proven to fall with increasing diameter. With this data, I was able to conclude that the outside mass transfer coefficient is the controlling resistance, because it caused the overall coefficient to follow its trend. This conclusion became evident because the coefficients for the outside mass transfer were greater in value, and because this term included the effect of the resistance induced by the discontinuity of the concentration of acetic acid among the two phases at the interface. The significance of this result is three-fold. First, the engineer can plan on his diameter size by realizing its effect on the overall mass transfer coefficient. This will help with his mass transfer equations in the design of the extraction unit operation. Second, and more importantly, this allowed us to see that the outside mass transfer coefficient is the controlling resistance. Thus, if the engineer has the ability to change any physical parameters (or maybe even materials) in his process to increase the mass transfer, he should ensure that the variables he is changing have the major effect on the outside mass transfer coefficient since it is this term that controls the process. Finally, realizing that the outside mass transfer coefficient is the controlling resistance, his time can wisely be spent modeling this factor accurately, and using this functionality as the basis for his scale-up calculations. Figure 12 is, also, the basis of answering our other objective concerning the overall mass transfer coefficient, namely, how well can we predict this value. It 59 was suggested by researchers that the model of Handlos and Baron, developed for general liquid-liquid extraction of a falling drop, grossly over predicts the overall mass transfer coefficient by 2 orders of magnitude. The reasoning for this was the instabilities at the surface of liquid-liquid extraction, such as surface tension, are significant, but not included in the original model for gas-liquid adsorption. However, as was pointed out by Henschke and Pfenning, the model can be easily adjusted to account for this extra instability by adding a constant instability factor in the denominator of both individual mass transfer coefficients. This value should remain constant for similar systems, though, it must be determined experimentally. For our system, I have determined the instability constant to be 113.28 with a possible error of 7%, which is in the range predicted for turbulent mass transfer systems. Addition of this factor improved our prediction to only 1.5% error. Thus, I have concluded that the model developed by Handlos and Baron with the adjustment by Henschke and Pfenning will work very well for predicting the trend of liquid-liquid extraction systems. This model can also be used with confidence for predicting absolute values. The only other requirement would be that the engineers run a small scale experiment in order to obtain a few data points to determine the instability factor. It is this value that will remain constant for the industrial extraction unit. My final objective was to determine the effect that diameter has on the overall mass transfer rate. This functionality can be predicted by looking at the factors controlling this transfer rate. From Newton’s relation, we know the factors of interest are the driving force, the surface area, and the overall mass transfer coefficient. We can immediately ignore the driving force because this value will not change with the diameter of the particle. Next, it should be fairly obvious that the surface area will increase with increasing diameter. Finally, we have already shown that the overall mass transfer coefficient decreases with increasing diameter. Thus we can say the functional relationship of the mass transfer rate will depend on which of these two factors is the controlling variable for our process. Table 7 reveals to us, from previous calculations that the surface area is 60 growing faster, relative to the decreasing of the overall mass transfer coefficient. From this information, we should expect the surface area to be the controlling factor and our mass transfer rate should increase. As evidenced by Figure 11, this dependency is noted. In this figure, we see the molar transfer rate increases almost linearly with an increase in diameter. Once again, I need to point to the significance of this result for engineering an extraction process. The large the engineer makes the diameter, the faster the particle will transfer mass. However, it is also important for him to note our previous functional relationship we found for the same particle’s terminal velocity. The particle is transferring mass at a higher rate, but it is also traveling much faster and has more mass to transfer before reaching an equilibrium state. Therefore to achieve equilibrium for larger drops, greater amounts of travel time and distance in the column is needed. These factors are essential and should be included in the design of the extraction column. In summary, when the engineer is designing an extraction column, he should take into consideration the following trends. First, that the terminal velocity of the particle and the mass transfer rate will both increase with an increase in diameter, while the overall mass transfer coefficient will decrease with an increase in diameter. The engineer should also note the following characteristics. One, the overriding individual mass transfer coefficient is that of the outside. Two, the overriding factor influencing the mass transfer rate is the surface area. Next, the engineer should realize that the distribution coefficient should lie in the vicinity of 10.63 (concentration of HAc in water to that in toluene), but that this value is concentration dependent. Also, he should note that the terminal velocity must be measured experimentally as the oscillations in the drop cause it to be far off the theoretical predictions but the theoretical trend predicted for the terminal velocity is accurate on a percentage basis. Finally, the model of Handlos and Baron, provides and excellent description of the trend of the overall mass transfer coefficient, but an experimentally determined instability factor is needed for accurate predictions of the absolute numbers. This factor should stay constant for all similar situations involving these materials. 61 The usefulness of these conclusions is evident in the design of extraction columns. The trends observed can be used in the rough sketch of the unit operation, while the predictive equations developed can be used to predict the column concentration profile and exit concentrations. Of course, to apply this, we have to change our assumption that the surface concentration and the internal drop concentration are both constant, as these values will vary with position in the column. Thus, an analytical solution would not be easy, an a finite difference algorithm is suggested, using the equations developed in this paper. 62 RECOMMENDATIONS: In this experiment, I determined, as did Henschke and Pfenning, that an experimentally determined instability constant allowed the modeling of the overall mass transfer coefficient to within 2% of the actual values. However, it was hard to determine for certain how accurate I was able to predict the value of this instability constant with only three data points. I recommend that the experimenter make up a known molarity of acetic acid in their toluene phase since we know that a molarity of .8 moles per liter acetic acid provides good results in this experiment, thus, freeing up some time in the second week for more runs to be made at different diameters. The benefits of this are that more data points can be run. This will better determine the accuracy of the instability constant by allowing it to be calculated through regression which allows the scatter to represent the error more accurately. The only negative concept that I encountered is that the titration of the toluene phase of the column is not required. In the process of determining the distribution coefficient, it is necessary to titrate a toluene phase and as a result the experience of doing technique is not lost. In fact, it eliminates the overkill of having to titrate this phase twice. 63 NOMENCLATURE: Abbreviations: A impurity component AQ aqueous phase E extract solvent HAc acetic acid ORG organic phase R raffinate phase T or Tol toluene or toluene phase W or Wat water or water phase Dimensionless Numbers: Nu Nusselt Number Pe Peclet Number Pr Prandtl Number Re Reynolds Number Sc Schmit Number Sh Sherwood Number # Dimensionless Group Variables: Projected Area m A surface area of drop m 2 AProj 2 m2 ag acceleration due to gravity sec mole Ccomponent i concentration of component i 3 m mole C * equilibrium concentration of HAc at surface 3 m CD drag coefficient CIP instability constant 64 D or d drop diameter m m2 DAB or DHAc fluid diffusivity sec F Force N m K overall mass transfer coefficient based on inside s m kin inside mass transfer coefficient s m kout outside mass transfer coefficient s M mass of drop kg MW molecular weight kg / kmole m distribution coefficient mole N mass transfer rate sec ni moles of component i mole p position in drop m r radius of drop m t time sec Vol or V volume m3 m vT terminal veloctiy sec z 2 expectation value of displacement m2 sec thermal diffusivity error eigen value kg m sec kg density 3 m viscosity 65 REFERENCES: 1. Bond and Newton. Philadelphia Magazine. 5,No. 7. Page 794. 1928 2. Fuse and Iguchi. Kagaku Kogaku. Experimental Tie Lines in Mole Percent of Acetic Acid in Toluene and Water. 35(1971)107. 3. Handlos and Baron. Mass and Heat Transfer from Drops in Liquid Liquid Extraction. AICHE Journal. Volume 3, No. 1. Pages 127-136. March 1957. 4. Henschke and Pfenning. Mass-Transfer Enhancement in Single Drop Extraction Experiments. AICHE Journal. Volume 45, No. 10. Pages 20792086. October 1999. 5. Higbie. Trans American Institute of Chemical Engineers. 31, 365 (1935). 6. Johnstone and Pigford and Chapin. Trans American Institute of Chemical Engineers. 37, 95 (1941). 7. Kronig and Brink. Applied Science Research. A2, 142 (1950). 8. Salerno, Tom. Convection Formal Report. June 2006. 9. Welty and Wicks and Wilson and Rorrer. Fundamentals of Momentum, Heat, and Mass Transfer – 4th Ed. John Wiley & Sons, Inc. New York. (2001) 10. West and Herrman and Chong and Thomas. Ind. Eng. Chem. 44, 621 (1952) 66 APPENDIX: Raw Data: During week one of this experiment, measurements of time were taken of the drop to travel certain distances in the column. This documented data was used to calculate the terminal velocity of the drop. The procedure and corresponding time measurements were done for each syringe size. Additionally during the first week, my group and I took measurements of the time needed for the drop to turn clear, aiding us in our calculation of the molar transfer rate. This data was also recorded for each corresponding syringe size. The raw numbers from these measurements are shown below in Tables 8, 9, and 10. 67 Large Syringe Calculating Volume Number of Drops Total Volume Volume per Drop Surface Area per Drop Radius of Drop 50 0.0000036 0.000000072 8.36962E-05 0.002580762 (quantity) (m^3) (m^3) (m^2) (m) Calculating Terminal Velocity Trial # 0-600 1 2 3 4 5 6 7 8 9 10 1.72 1.72 1.71 1.57 1.59 1.68 1.7 1.73 1.72 1.64 1.678 Average Time (s) Length per 100 marks Length per 600 marks Average Velocity 600-1200 1200-1800 1.94 1.84 2.08 1.8 1.85 1.74 1.86 1.82 1.8 1.81 1.92 1.84 1.73 1.83 1.86 1.85 1.97 1.86 1.77 1.84 1.878 1.823 0.0355 (m) 0.213 (m) 0.118795315 (m/s) 0.001775 Calculating Mass Transfer Rate Trial # 1 2 3 4 5 6 7 8 9 10 11 Average time (s) Concentration of NaOH Moles of NaOH in drop Moles of HAc transferred N - transfer rate K overall - exp Distance 1 5.36 5.37 5.33 5.52 5.65 5.43 5.6 5.38 5.74 5.74 5.89 5.578636364 0.01 0.00000072 0.00000072 1.29064E-07 Distance 2 5.76 5.43 5.44 5.64 5.05 5.83 5.53 5.81 5.74 5.57 5.92 (m) M moles moles mole/s 1.86851E-07 Table 8: Raw Data, Week 1, Large Syringe 68 Medium Syringe Calculating Volume Number of Drops Total Volume Volume per Drop Surface Area per Drop Radius of Drop 50 0.00000165 0.000000033 4.97539E-05 0.001989795 (quantity) (m^3) (m^3) (m^2) (m) Calculating Terminal Velocity Trial # 0-600 1 2 3 4 5 6 7 8 9 10 Average Time (s) Length per 100 marks Length per 600 marks Average Velocity 2.01 1.97 1.97 1.9 1.95 1.95 1.95 1.96 2.01 2.08 1.975 600-1200 1200-1800 2.01 2.16 1.92 2.01 2.27 2.07 2.15 2.09 2.33 1.93 2.33 2.05 2.39 2.01 2.11 2.02 2.24 2.07 2.21 2.06 2.196 2.047 0.0355 (m) 0.213 (m) 0.102766163 (m/s) Calculating Mass Transfer Rate Trial # 1 2 3 4 5 6 7 8 9 10 11 Average time (s) Concentration of NaOH Moles of NaOH in drop Moles of HAc transferred N - transfer rate K overall - exp Distance 1 4.34 3.91 4.3 4.18 4.48 3.98 4.07 4.01 4.01 3.96 4.49 4.177272727 0.01 0.00000033 0.00000033 7.89989E-08 Distance 2 4.41 4.14 4.05 3.98 4.56 4.06 4.06 4.06 4.11 4.54 4.2 (m) M moles moles mole/s 1.92393E-07 Table 9: Raw Data, Week 1, Medium Syringe 69 Small Syringe Calculating Volume Number of Drops Total Volume Volume per Drop Surface Area per Drop Radius of Drop 60 0.0000013 2.16667E-08 3.75848E-05 0.001729423 (quantity) (m^3) (m^3) (m^2) (m) Calculating Terminal Velocity Trial # 0-600 1 2 3 4 5 6 7 8 9 10 Average Time (s) Length per 100 marks Length per 600 marks Average Velocity 2.06 2.26 2.12 2.34 2.12 2.24 2.18 2.25 2.1 2.22 2.189 600-1200 1200-1800 2.38 2.5 2.34 2.23 2.41 2.13 2.42 2.17 2.28 2.24 2.32 2.12 2.37 2.27 2.13 2.3 2.39 2.1 2.26 2.3 2.33 2.236 0.0355 (m) 0.213 (m) 0.094596595 (m/s) Calculating Mass Transfer Rate Trial # Distance 1 1 2 3 4 5 6 7 8 9 10 11 3.7 3.67 3.61 3.46 3.56 3.73 4.12 3.52 3.53 3.24 Average time (s) Concentration of NaOH Moles of NaOH in drop Moles of HAc transferred N - transfer rate 3.576 0.01 2.16667E-07 2.16667E-07 6.05891E-08 K overall - exp 1.95334E-07 Distance 2 3.61 3.71 3.61 3.6 3.3 3.6 3.63 3.67 3.21 3.44 (s) M moles moles mole/s Table 10: Raw Data, Week 1, Small Syringe 70 In week two of this experiment, the necessary titrations were performed by the group. This data allowed us to immediately calculate the concentration of acetic acid in the toluene phase and the equilibrium distribution coefficient at this concentration of acetic acid in the toluene phase. This data is shown below in Table 11. Titration 1: Calculating HAc in Toluene Amount of T solution added Amount of .1M NaOH added Amount of .1M HCl added Amount of HAc present Concentration of HAc in bulk 0.005 0.0506 0.0131 0.00375 0.75 (L) (L) (L) moles mol/L 0.005 0.05 0.0099 0.00401 0.802 (L) (L) (L) moles mol/L should make a strong pink color back titrated to light pink fading endpoint Titration 2: Calculating HAc in Toluene Amount of T solution added Amount of .1M NaOH added Amount of .1M HCl added Amount of HAc present Concentration of HAc in bulk should make a strong pink color back titrated to light pink fading endpoint Titration Avg: Calculating HAc in Toluene should make a strong pink color back titrated to light pink fading endpoint Concentration of HAc in bulk Stdev Concentration of HAc in bulk 0.776 mol/L 0.036769553 mol/L Calculating Distribution Coefficient Amount of pure T added Amount of pure W added Estimated amount of Hac needed: 0.05 L 0.05 L Concentration of HAc needed in T Concentration of HAc in T Moles HAc need in T Volume of HAc in T Est. Distribution Coeff Concentration of HAc needed in W Concentration of HAc in W Moles HAc need in W Volume of HAc in W 0.03677 0.800085 0.041923 0.002398 10 8.000854 8.000198 0.737393 0.042172 mol/L mol/L mol L Total moles HAc needed Total volume HAc needed 0.779316 mol 0.044569 L Amount of T soln added Amount of .1M NaOH added Amount of .1M HCl added Amount of HAc present Concentration of HAc in T Phase 0.005 0.0402 0.00005 0.004015 0.803 L L L moles mol/L Amount of W soln added Amount of .1M NaOH added Amount of .1M HCl added Amount of HAc present Concentration of HAc in T Phase 0.0005 0.0427 0 0.00427 8.54 L L L moles mol/L mol/L mol/L mol L Titrating Toluene Phase: Titrating Water Phase: m - Distribution Coefficient orig est m - Distribution Coefficient 10 10.63511831 C* - conc of HAc at interface C* - conc of HAc at interface 8.252851806 mol/L 8252.851806 mol/m^3 K - overall mass trans coeff 1.86851E-07 Table 11: Raw Data, Week 2, Titrations 71 Sample Calculations: Experimental: During the first week of the experiment, I calculated the terminal velocity of my falling drop. As described previously in my report, this involved taking numerous measurements of the time needed for the drop to pass through .213 meter sections of the column. Starting with the large drop, in order to find the average velocity, I first had to calculate the average time for the particle to pass each .213 meter section. This was accomplished by adding up each measurement unit and dividing by the total number of units. The data received from this part is displayed in Table 8. Mathematically this is calculated as, 30 Average time (67) Average time time i 1 i 30 1.62 1.72 1.71 1.67 1.59 1.68 1.7 1.73 1.72 1.64 1.84 2.08 1.85 1.86 1.8 1.92 1.73 1.86 1.97 1.87 1.84 1.8 1.78 1.82 1.81 1.84 1.83 1.85 1.82 1.84 30 Average time 1.793 sec Next, I calculated the average velocity by dividing the .213 meter distance that each time was recorded with by the average time, this gave me the following result, (68) v Distance Traveled .213 m .1188 m / sec Average Time 1.793 sec The error measurement in this calculation is derived from the error in measuring the distance and the time. Since I used a ruler that had markings every millimeter, it can be assumed that the error in measuring the distance to be half a millimeter.. The error in the time can be assumed to be the scatter in the time measurements, 72 or more specifically the standard deviation of the time measurements, which is calculated by, 30 (69) std dev Time measurment i i 1 Average Time 2 30 .105 sec Thus we can calculate the error in our measured velocity as, error in distance std dev in time distance time .0005m .105sec v 6.1% .213m 1.793sec v (70) After this was completed, I needed to find the molar transfer rate from the large drop. In order to do this, as described previously, I needed to take time measurements starting from the time the drop entered the toluene phase, and ending when the drop turned clear in the toluene phase. This data allowed me to ascertain the time needed for the acetic acid to diffuse into the drop and neutralize the entire amount of NaOH present. Thus, the first step in finding the value of this variable was to find out how much NaOH was present. To do this I needed two important statistics, the concentration of NaOH in the water phase, which is known to be .01M and the volume of the drop of the water phase from the large syringe. To calculate the volume of each drop my group and I measured the volume of 50 of drops, and then divided the total volume by this amount. My group and I proceeded to drop 50 drops of our water phase solution into a 10ml graduated cylinder, and measured its volume to be 0.0000036m3. We can calculate the volume per drop from, (71) Vdrop Total Volume 0.0000036m3 7.2e8 m3 / drop # of drops 50 drops 73 This data allowed us to calculate the average amount of NaOH present in each drop from, (72) nNaOH , Wat Vdrop * CNaOH , Wat 7.2e 8 m3 * 1000 L mole *.01 7.2e 7 mole NaOH m3 L The next step was to calculate how much acetic acid needed to enter the drop to neutralize the 7.2e7 mole NaOH present. To attain this, we need to look at the neutralization reaction between these compounds given to us by Equation 3, (3) CH 3CH 2COOH (aq) NaOH (aq) H 2O(l ) CH 3CH 2COO Na (aq ) From this equation, you can see that there is a 1:1 relationship between NaOH and acetic acid in the neutralization reaction. Hence, the amount of acetic acid that gets transferred into the water phase while the drop is still colored is equal to the amount of NaOH originally present which we calculated from Equation 70. Now that we know how much was entered, we need to know the time required for this phase in order to find the mass transfer rate. This requires us to calculate the average of the measurements of the time it took for this drop to turn clear. This can be done by, 22 taverage to turn clear (74) taverage to turn clear taverage to turn clear time measurement i 1 i 22 5.36 5.37 5.33 5.52 5.65 5.43 5.6 5.38 5.74 5.74 5.89 5.76 5.43 5.44 5.64 5.05 5.83 5.53 5.81 5.74 5.57 5.92 22 5.579sec Now, we can calculate the molar transfer rate as, 74 N nNaOH , Wat amount HAc diffused average time taverage to turn clear N 7.2e7 mole 1.29e7 mole / sec 5.58 sec (75) Next, we can calculate the error involved in the experimental measurement of this value. This error is derived from the error in predicting the amount of NaOH in the drop, which comes from the error in finding the volume, the error in the molarity, and the standard deviation in the time measurements. The error in the volume depended largely upon the ability of our group to read the volume of the 50 drops, which can be assumed to be .1ml since the graduated cylinder had markings every .2mL. The error in the molarity of the solution must be estimated, since this molarity is very small, a 5% error isn’t unlikely. The standard deviation in the time can be estimated as shown in Equation 69 and is found to be .217 seconds. Thus, we can calculate the error in the molar transfer rate as follows, error in volume error in molarity std dev in time volume molarity time 1 .1mL .217 sec N 50 5% 11.7% 1 5.579sec 3.6mL 50 N (76) In week two, my group and I titrated the toluene phase. This involved taking 5ml of my toluene phase and placing it in a stirred beaker. An excess of NaOH was added to ensure that all of the acetic acid present was neutralized. It was essential to add excess because the two phases are immiscible. Then, we had to back titrate with HCl to reach the phenolphthalein endpoint. As described in the procedure section of this report, the calculation procedure to determine the amount of acetic acid present in the toluene phase is, 75 mmole HAc mL NaOH mL HCl .1 mmole mL mmole HAc .0506mL NaOH .0311mL HCl .1 mmole mL mmole HAc 3.75 mmole HAc CHAc , Tol 5 mL sample 3.75 CHAc , Tol .75mol / L HAc 5 (77) This procedure was then repeated and resulted in CHAc , Tol .802mol / L HAc . Thus, the average value was taken as our actual concentration from, CHAc , Tol (78) .75 .802 mol / L HAc .776mol / L HAc 2 The error involved in this measurement can be derived from a number of sources. First, we have to estimate the amount of NaOH and HCl in the titrating columns both before titration and after titration. There is an error in estimating the 5ml of sample that needed to be taken from my toluene phase. Finally, there is an error in judging when the solution has reached its phenolphthalein endpoint. Because of the numerous possible errors and the uncertainty in estimating the latter mentioned error source, the measurement error in the value of Equation 78 was calculated from the standard deviation of the two data points taken. Which is written, .75 .776 .802 .776 2 std dev, CHAc , Tol (79) CHAc , Tol std dev, CHAc , Tol CHAc , Tol 2 .0368 4.7% .776 2 .0368 . My group and I recognize that using the standard deviation of only two points isn’t a true measure of the error involved; however, our answer of 4% error with 76 the number of sources involved seems to be in the range of percent error we would have expected. Therefore, we accept this as our error. Next, we had to determine the distribution coefficient of acetic acid in toluene and water at .776mol / L HAc in Tol . Following the calculation steps described in the procedure section of this report, we have to estimate the amount of acetic acid to add in order to give us this concentration of acetic acid in our toluene phase. This is performed, mmole 40 mL mmole mmole HAc in Wat 50mL Wat *10*.8 400 mL mmole HAc mmole HAc in Tol mmole HAc in Wat 440 mole 60.05 g 1mL mL HAc mmole HAc * * * 1000mmole mole 1.05 g mole 60.05 g 1mL mL HAc 440 mmole HAc * * * 25.2mL HAc 1000mmole mole 1.05 g mmole HAc in Tol 50 mL Tol * .8 (80) Approximately 50ml of both toluene and water and approximately 26 ml of acetic acid were added into a separatory funnel and mixed. We titrated each via the procedure already described, and performed the calculations by the procedure already described to find that (81) m CHAc , Wat CHAc , Tol 8.54mol / L 10.64 .803mol / L The error in this calculation is derived again by the error involved in making the titrations, which we have defined to comprise numerous sources. We would have liked to take the standard deviation of a number of measurements of this value, however, time did not allow us to obtain another measurement of this value. Thus, we had to assume that our titration ability is relatively consistent, such that we would incur the same error in each of these titrations. The error in our distribution coefficient would then be, 77 2 (82) m Percent Error in Titration i 2* 4.7% i 1 m 9.4% Theoretical: Our next task was to calculate predictions of these experimental values based from theory. From our force balance, performed in the theory section of this report, we find our velocity depends on time according to the following differential equation, which has been set equal to zero since we are finding the terminal velocity, i.e., the velocity at which the velocity no longer depends on time. (20) 2 dv agravity Wat Tol CD v v Tol * rdrop 0 dt Wat 2* M Our first step in solving Equation 20 is to ensure the only variable in the equation is the unknown velocity. In order to replace the drag coefficient with a function that depends on velocity alone, we have the following correlations to choose from: (21a) CD (21b) CD (21c) 24 for Re 2 Re 18.5 for 2 Re 500 Re.6 CD .44 for Re 500 . For our large particle, we will assume that the velocity will be large enough to give us a Reynolds number above 500. The major reasoning of this prediction was so that as our first guess, we will simply have to plug in a constant for C D into our equation instead of a function form of the Reynolds number which depends on velocity. Performing this we arrive at 78 (83) v agravity Wat Tol 2* M Wat 2 .44* Tol * rdrop Next we can replace the unknown mass term with the volume of the drop times the density, which we both know. We now have (84) 4 3 agravity Wat Tol 2* Wat 3 rdrop . v 2 Wat .44* Tol * rdrop Plug in all of the known values into this equation and solve for the terminal velocity. (85) 4 3 8 1 agravity Wat Tol 2* Wat 3 rdrop agravity Wat Tol 3 Wat rdrop v 2 Wat .44* Tol * rdrop Wat .44 Tol 2 v 8 3 867 kg / m3 3 *998kg / m * .00258m .152m / sec 998kg / m3 .44*867 kg / m3 9.81m / sec 998kg / m 3 Our next step will be to determine the error in this calculation. Since all terms are multiplied, we can set up our error equation as (86) v 1 a 1 Wat Tol 1 Wat 1 rdrop . v 2 a 2 Wat Tol 2 Wat 2 rdrop In this equation, we can assume the error due to the acceleration due to gravity is only 1%, because we had numerous measurements and calculations of this value in literature. The error in the density can be gathered by averaging all of the values listed for the densities in many literature sources and finding the standard deviation of this error. The results of this allowed us to find the error due to the density was approximately 1.06% . Finally, the error in the radius of the drop has 79 to be calculated. We calculated the radius of the drop by measuring the volume of 50 drops, calculating the volume of one drop and finally the average radius of each drop. The error in this calculation is (87) rdrop rdrop 1 .1mL 50 2.78% 1 3.6mL 50 Thus, we can calculate our error in predicted velocity to be v 1 a 1 Wat Tol 1 Wat 1 rdrop v 2 a 2 Wat Tol 2 Wat 2 rdrop (88) v 1 1 1 1 *1% * 2*1.06% *1.06% * 2.8% v 2 2 2 2 v 3.5% v The experimental error can now be calculated by the difference of the terminal velocity measured in week 1 and the predicted value calculated in Equation 85. Exp Error (89) Exp Error vT , exp vT , pred vT , pred *100% .119 .152 *100% 22.0% .152 To determine the predicted value of the distribution coefficient, my group and I used recorded experimental data given to us by Fuse and Iguchi. However, their data is in mole percent, whereas, our experimental data is in weight percent, thus it became necessary to translate my data into mole percent to compare. This task can be accomplished by first assuming we have 1000mL of both toluene and water, of which we have determined their concentration to be .803 and 8.54 80 mol per liter respectively. Thus to calculate the mole percent of HAc in the toluene phase we have, mL Tol phase 1000mL mole HAc in Tol 1L *.803mol / L .803mole .803mol *60.01g / mole mL HAc in Tol 1L 45.89mL 1.05 g / L mL Tol 1L 45.89mL 954.11mL (90) g 954.11mL *.867 954.11mL * Tol L 8.98mole mole Tol MWTol g 92.14 mol .803 mole% HAc in Tol .0821 .803 8.98 Similarly we can calculate the mole percent of HAc in the water phase to be, mL Wat phase 1000mL mole HAc in Wat 1L *8.54mol / L 8.54mole 8.54mol *60.01g / mole mL HAc in Wat 1L 488mL 1.05 g / L mL Wat 1L 488mL 512mL (91) g 512mL *.999 512mL * Wat L 28.416mole mole Wat g MWWat 18 mol 8.54 mole% HAc in Wat .231 8.54 28.416 Thus our experimental distribution coefficient in mole percent turns out to be (92) mole percent HAc in Wat phase .231 mole percent HAc in Tol phase .0821 2.82 mmole mmole 81 Which is close (only 18.6% difference) to 2.32, the experimental found by Fuse and Iguchi in their research. Next, my group and I had to calculate the predicted values for the individual mass transfer coefficients. As noted earlier, the velocity used for these equations is the experimental velocity since this was determined to be a better estimate of the true velocity. The calculation of these are done by, koutside 1.13 1/ 2 vT1/ 2 DHAc 2 r 1/ 2 drop (93) .119 2.26e9 koutside 1.13 1/ 2 2 .00258 1/ 2 1/ 2 2.57e 4 .00375vT i 1 o .00375 .119 ki 1.8e 4 .001 1 .00068 ki (94) Next we had to calculate the overall mass transfer coefficient. This was achieved by graphing the equilibrium between the two phases and plotting the driving forces. The calculation procedure is as follows, 1 1 m K kin kout (95) 1 1 10.635 4 2.57e 4 K 1.8e K 2.14e 5 It was then determined that the equations presented by Handlos and Baron required an experimentally determined instability factor to be added. The predictive equations took the form, 82 (54) Shi .00375Pe CIP 1 i o Shoutside (55) koutside koutside 2 rdrop DHAc 1/ 2 1.13Peoutside 1.13 vT 2 rdrop CIP CIP DHAc 1/ 2 1/ 2 1.13 vT1/ 2 DHAc CIP 2 r 1/ 2 drop In order to determine this coefficient, we need only graph the K from experiment versus that from predicted. The slope of this graph gave us CIP. Predicting the Instability Constant 224 K pred orig (10^-7 m/s) 222 220 218 y=111.11*Kexp+6.07 216 214 212 1.86 1.87 1.88 1.89 1.9 1.91 1.92 1.93 1.94 1.95 K exp (10^-7 m/s) Figure 13: Graph of Kpred versus Kexp to determine CIP From Figure 13, we have estimated our CIP constant to be 113.28. The error involved from this calculation can again be associated to the scatter in the data, which is 83 1.96 (96) CIP 7.0% . The experimental error can be calculated as done before in Equation 89. This concludes a sample of each type of calculation for the liquid-liquid extraction, falling drop experiment. THESE CALCULATIONS HAVE BEEN REVIEWED AND APPROVED BY: THOMAS SALERNO: _______________________ GREGORY ROTHSCHING: _______________________ AN DU: _______________________ 84