Chemistry - College of Education
advertisement

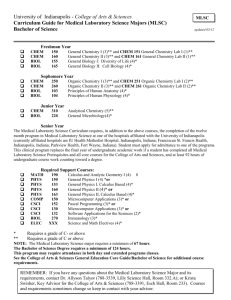
Minnesota State University, Mankato Professional Education Unit INITIAL LICENSURE PROGRAM UNDER the LICENSURE RULES (LAWS OF MINNESOTA; 1993, CHAPTER 224, ARICLE 12, SECTION 34) College: Science, Engineering & Technology Department: Chemistry & Geology Submission Date to BOT: July 1, 2000 Studies Program: Chemistry (9-12) Degree: BST Licensure Standards: Minnesota Board of Teaching Standards Date: March 1999 Program Contact Person(s): Jeffrey R. Pribyl Licensure Rule 8710.4750 Standards Knowledge and Understanding of the Standard Course Numbers and Titles Practice in Applying the Standard CHEM 202: General Chemistry II CHEM 202: General Chemistry II Course Numbers and Titles Assessment (Demonstration of Attainment of the Standard) Course Numbers and Titles A. A teacher of chemistry must demonstrate a conceptual understanding of chemistry. The teacher must: (1) use sources of information to solve unfamiliar quantitative problems and communicate the solution in a logical and organized manner as evidenced by the ability to: (a) describe, in terms of the known and unknown quantities, a given problem in appropriate pictorial, graphical, or written forms; CHEM 202: General Chemistry II 1 Licensure Rule 8710.4750 Standards (b) describe, in terms of the relevant numerical and algebraic quantities and equations, a given problem mathematically; (c) plan, using words, diagrams, and mathematical relationships, a solution for a given problem in terms of steps necessary to solve the problem and to verify the solution; and (d) evaluate, in terms of unit consistency, reasonableness, and completeness of solution, the solution of a given problem; (2) use computers to display and analyze experimental and theoretical data as evidenced by the ability to: (a) describe data graphically using a computer; and (b) design a mathematical model to provide a reasonable fit to a given set of data; and (3) develop a plan to ensure a safe environment and practices in chemistry learning activities. B. A teacher of chemistry must demonstrate a knowledge of chemistry concepts. The teacher must: (1) understand the properties and structure of matter as evidenced by the ability to: (a) explain and predict, using the principles for filling the electron orbitals of atoms and the Periodic Table, the periodic trends in electrical conductivity, atomic, radii, ionization energy, Knowledge and Understanding of the Standard Course Numbers and Titles Practice in Applying the Standard CHEM 202: General Chemistry II CHEM 202: General Chemistry II CHEM 202: General Chemistry II CHEM 202: General Chemistry II CHEM 202: General Chemistry II CHEM 202: General Chemistry II CHEM 202: General Chemistry II CHEM 202: General Chemistry II CHEM 202: General Chemistry II CHEM 440: Physical Chemistry I CHEM 450: Physical Chemistry Lab I CHEM 440: Physical Chemistry I CHEM 450: Physical Chemistry Lab I CHEM 440: Physical Chemistry I CHEM 450: Physical Chemistry Lab I CHEM 381: Introduction to Research CHEM 440: Physical Chemistry I CHEM 450: Physical Chemistry Lab I CHEM 440: Physical Chemistry I CHEM 450: Physical Chemistry Lab I CHEM 440: Physical Chemistry I CHEM 450: Physical Chemistry Lab I CHEM 381: Introduction to Research CHEM 440: Physical Chemistry I CHEM 450: Physical Chemistry Lab I CHEM 440: Physical Chemistry I CHEM 450: Physical Chemistry Lab I CHEM 440: Physical Chemistry I CHEM 450: Physical Chemistry Lab I CHEM 381: Introduction to Research CHEM 412: Intermediate Inorganic Chemistry CHEM 412: Intermediate Inorganic Chemistry CHEM 412: Intermediate Inorganic Chemistry Course Numbers and Titles Assessment (Demonstration of Attainment of the Standard) Course Numbers and Titles 2 Licensure Rule 8710.4750 Standards electronegativity, electron affinity, and metallic character of a given set of elements; (b) predict, using the Periodic table and the arrangement and energies of the element’s outermost electrons, whether the bonding in a given substance is primarily covalent, metallic, or ionic; (c) explain and predict, using the periodic trends in the physical and chemical characteristics of the elements and the type of bonds, or intermolecular forces, or both, the relative magnitudes of a given property for a set of elements or compounds; (d) predict, using existing models including the Valence Shell electron Pair Repulsion theory, the shape of a given molecule; and (e) describe, with words and diagrams using neutron to proton ratios and binding energies, the changes in matter and energy that occur in the nuclear processes of radioactive decay, fission, fusion, and other common nuclear transformations; (2) understand chemical reactions as evidenced by the ability to: (a) perform measurements and calculations to determine the chemical formulas of the products of a given chemical reaction; (b) explain and predict qualitatively and quantitatively, using the Periodic Table and the concept of chemical stoichiometry, the mass relationships between reactants and products for a Knowledge and Understanding of the Standard Course Numbers and Titles Practice in Applying the Standard CHEM 201: General Chemistry I CHEM 201: General Chemistry I CHEM 201: General Chemistry I CHEM 201: General Chemistry I CHEM 201: General Chemistry I CHEM 201: General Chemistry I CHEM 201: General Chemistry I CHEM 201: General Chemistry I CHEM 201: General Chemistry I CHEM 202: General Chemistry II CHEM 202: General Chemistry II CHEM 202: General Chemistry II CHEM 201: General Chemistry I CHEM 440: Physical Chemistry Lab II CHEM 201: General Chemistry I CHEM 440: Physical Chemistry Lab II CHEM 412: Intermediate Inorganic Chemistry CHEM 440: Physical Chemistry Lab II CHEM 412: Intermediate Inorganic Chemistry CHEM 440: Physical Chemistry Lab II Course Numbers and Titles Assessment (Demonstration of Attainment of the Standard) Course Numbers and Titles 3 Licensure Rule 8710.4750 Standards given chemical reaction; (c) predict quantitatively, using the principle of state functions and Hess’s Law, the molar heat of a given reaction from known values of molar heats of formation or molar heats of a series of related reactions; and (d) explain and predict qualitatively and quantitatively, using solubility rules, the common oxidation states of elements, the activity series of metals and nonmetals, stability of radicals, and the properties of acids and bases, the most likely type of reaction for a given set of given reactants; (3) understand thermodynamics as evidenced by the ability to: (a) perform measurements and calculations to determine the molar heat energy absorbed or released in a given phase change or chemical reaction; (b) predict qualitatively and quantitatively, using the Ideal Gas Law, changes in the pressure, volume, temperature, or quantity of gas in a given thermally isolated ideal gas system when the gas is heated or cooled, is compressed or expanded adiabatically, or enters or leaves the system; (c) describe, using words, diagrams, energy graphs, and mathematical relationships, the changes in the enthalpy, entropy, and Gibb’s free energy during a given chemical reaction; (d) explain and predict qualitatively and quantitatively, using the First and Second laws of Thermodynamics and the Knowledge and Understanding of the Standard Course Numbers and Titles Practice in Applying the Standard CHEM 201: General Chemistry I CHEM 440: Physical Chemistry I CHEM 201: General Chemistry I CHEM 440: Physical Chemistry I CHEM 201: General Chemistry I CHEM 440: Physical Chemistry I CHEM 201: General Chemistry I CHEM 412: Intermediate Inorganic Chemistry CHEM 412: Intermediate Inorganic Chemistry CHEM 201: General Chemistry I CHEM 440: Physical Chemistry I CHEM 440: Physical Chemistry I CHEM 201: General Chemistry I CHEM 201: General Chemistry I CHEM 440: Physical Chemistry I CHEM 201: General Chemistry I CHEM 440: Physical Chemistry I CHEM 202: General Chemistry II CHEM 440: Physical Chemistry I CHEM 440: Physical Chemistry I CHEM 440: Physical Chemistry I CHEM 202: General Chemistry II CHEM 202: General Chemistry II CHEM 440: Physical Chemistry I CHEM 440: Physical Chemistry I Course Numbers and Titles Assessment (Demonstration of Attainment of the Standard) Course Numbers and Titles 4 Licensure Rule 8710.4750 Standards relationship between Gibb’s free energy and the equilibrium constant, changes in the equilibrium and Gibb’s free energy for a given change in the reaction conditions; (e) design, using Gibb’s free energy, a method for changing the direction of spontaneity of a given reaction; and (f) explain qualitatively and quantitatively, using Gibb’s free energy, how the electrochemical potential of a given cell depends on given changes in the temperature or the concentration of ions in solution, or both; (4) understand chemical kinetics and equilibrium as evidenced by the ability to: (a) perform measurements and calculations to determine the rate of a chemical reaction, the rate expression, half-life of given reaction, the activation energy of a given reaction, and the equilibrium constant of a given reaction; (b) describe, using words, energy diagrams, graphs, and mathematical relationships, the activation energy, enthalpy changes, and reaction rate of a given reaction; (c) explain and predict qualitatively and quantitatively, using the rate equation for the reaction, changes in the reaction rate for a given change in the concentration of a reactant or product; (d) predict, using the rate equation and the presence or absence of intermediates, a possible mechanism for a given reaction; Knowledge and Understanding of the Standard Course Numbers and Titles Practice in Applying the Standard CHEM 202: General Chemistry II CHEM 202: General Chemistry II CHEM 440: Physical Chemistry I CHEM 440: Physical Chemistry I CHEM 202: General Chemistry II CHEM 202: General Chemistry II CHEM 440: Physical Chemistry I CHEM 440: Physical Chemistry I CHEM 202: General Chemistry II CHEM 440: Physical Chemistry I CHEM 450: Physical Chemistry Laboratory II CHEM 202: General Chemistry II CHEM 450: Physical Chemistry I CHEM 202: General Chemistry II CHEM 450: Physical Chemistry Laboratory II CHEM 202: General Chemistry II CHEM 202: General Chemistry II CHEM 202: General Chemistry II CHEM 450: Physical Chemistry Lab II CHEM 202: General Chemistry II CHEM 320: Organic Chemistry I CHEM 320: Organic Chemistry I CHEM 320: Organic Chemistry I Course Numbers and Titles Assessment (Demonstration of Attainment of the Standard) Course Numbers and Titles 5 Licensure Rule 8710.4750 Standards (e) describe, using words, diagrams, chemical equations, concentration and rate graphs, and mathematical relationships, the equilibrium of a given reaction; (f) explain, in terms of changes in the number of effective collisions of the molecules in the forward and reverse reaction, why the chemical equilibrium of a given reaction is a dynamic process; (g) explain and predict quantitatively, using the equilibrium constant, the concentration of a reactant or product in a given gas phase or solution chemical reaction; (h) design, using LeChatelier’s principle, a method for achieving a specified change in the equilibrium constant or the position of equilibrium of a given chemical reaction; and (i)design, using the rate laws and requirements for effective collisions, a method for achieving a specified change in the rate of a given chemical reation; and (5) understand organic and biochemical reactions as evidenced by the ability to: (a) perform measurements and calculations to determine the melting point, boiling point, solubility, or other common physical properties of an organic compound; (b) describe, using words, structural and chemical formulas, and physical and computer models, the functional groups and polarity of the molecule of a given organic compound; Knowledge and Understanding of the Standard Course Numbers and Titles CHEM 202: General Chemistry II Practice in Applying the Standard Course Numbers and Titles CHEM 202: General Chemistry II Assessment (Demonstration of Attainment of the Standard) Course Numbers and Titles CHEM 202: General Chemistry II CHEM 202: General Chemistry II CHEM 202: General Chemistry II CHEM 450: Physical Chemistry Lab II CHEM 202: General Chemistry II CHEM 450: Physical Chemistry Lab II CHEM 202: General Chemistry II CHEM 202: General Chemistry II CHEM 440: Physical Chemistry I CHEM 440: Physical Chemistry I CHEM 202: General Chemistry II CHEM 202: General Chemistry II CHEM 440: Physical Chemistry I CHEM 202: General Chemistry II CHEM 202: General Chemistry II CHEM 450: Physical Chemistry Lab II CHEM 450: Physical Chemistry Lab II CHEM 320: Organic Chemistry I CHEM 320: Organic Chemistry I CHEM 320: Organic Chemistry I CHEM 320: Organic Chemistry I CHEM 320: Organic Chemistry I CHEM 320: Organic Chemistry I 6 Licensure Rule 8710.4750 Standards (c) describe, using words, structural and chemical formulas, and physical or computer models, a given hydrocarbon compound as aromatic or aliphatic; saturated or unsaturated; alkanes, alkenes, or alkynes; and branched or straight chains; (d) explain and predict, using a molecular orbital model of the pi-bond, the outcomes or reactions of given aromatic, allylic and conjugated alkenes, and other delocalized electron systems; (e) explain and predict, using functional groups, structure, and polarity, the reactivity, solubility, melting point, and boiling point of an organic compound; (f) predict, using infrared, nuclear magnetic resonance, and mass spectra, the structure of an organic molecule; (g) design and carry out a single step synthesis of an organic compound, purify the compound, and characterize the product; (h) describe, using words, diagrams, structural and chemical formulas, and physical and computer models, the origin of optical activity of a given chiral organic compound; (i ) explain why the reactivity of a chiral compound depends on its stereo chemistry when acted upon by a living system, and predict whether a particular substrate enantiomer would or would not react with its enzyme; (j) describe, using words, structural and chemical formulas, and physical and Knowledge and Understanding of the Standard Course Numbers and Titles CHEM 320: Organic Chemistry I Practice in Applying the Standard Course Numbers and Titles CHEM 320: Organic Chemistry I Assessment (Demonstration of Attainment of the Standard) Course Numbers and Titles CHEM 320: Organic Chemistry I CHEM 320: Organic Chemistry I CHEM 320: Organic Chemistry I CHEM 320: Organic Chemistry I CHEM 320: Organic Chemistry I CHEM 320: Organic Chemistry I CHEM 320: Organic Chemistry I CHEM 320: Organic Chemistry I CHEM 320: Organic Chemistry I CHEM 320: Organic Chemistry I CHEM 320: Organic Chemistry I CHEM 320: Organic Chemistry I CHEM 320: Organic Chemistry I CHEM 320: Organic Chemistry I CHEM 320: Organic Chemistry I CHEM 320: Organic Chemistry I CHEM 360: Principles of Biochemistry CHEM 360: Principles of Biochemistry CHEM 360: Principles of Biochemistry CHEM 360: Principles of Biochemistry CHEM 360: Principles of Biochemistry CHEM 360: Principles of Biochemistry 7 Licensure Rule 8710.4750 Standards computer models, a given set of biomolecules as a carbohydrate, lipid, protein, or nucleic acid, and explain how biomolecules are made from typical chemical components by chemical reactions; (k) perform tests and measurements to determine if a given biological substance is a carbohydrate, lipid, protein, or nucleic acid; (l) explain, using the concepts of electrostatic attraction, repulsion, and stereochemistry in the catalytic process, how enzymes facilitate a given biochemical reaction; and (m) design a method to use organic compounds to demonstrate a given general chemical principle. C. A teacher of chemistry must demonstrate an advanced conceptual understanding of chemistry and the ability to apply its fundamental principles, laws, and concepts by completing a full research experience. The teacher must: (1) identify various options for a research experience including independent study projects, participation in research with an academic or industry scientist, directed study, internship, or field study; (2) select an option and complete a research experience that includes conducting a literature search on a problem; (3) design and carry out an investigation; Knowledge and Understanding of the Standard Course Numbers and Titles Practice in Applying the Standard CHEM 360: Principles of Biochemistry CHEM 360: Principles of Biochemistry CHEM 360: Principles of Biochemistry CHEM 360: Principles of Biochemistry CHEM 360: Principles of Biochemistry CHEM 360: Principles of Biochemistry CHEM 320: Organic Chemistry I CHEM 320: Organic Chemistry I CHEM 320: Organic Chemistry I CHEM 381: Introduction to Research CHEM 381: Introduction to Research CHEM 381: Introduction to Research CHEM 381: Introduction to Research CHEM 381: Introduction to Research CHEM 381: Introduction to Research CHEM 495: Senior Seminar CHEM 495: Senior Seminar CHEM 495: Senior Seminar Course Numbers and Titles Assessment (Demonstration of Attainment of the Standard) Course Numbers and Titles 8 Licensure Rule 8710.4750 Standards (4) identify modes for presenting the research project; and (5) present the research project in the selected mode. Knowledge and Understanding of the Standard Course Numbers and Titles CHEM 495: Senior Seminar Practice in Applying the Standard Course Numbers and Titles CHEM 495: Senior Seminar Assessment (Demonstration of Attainment of the Standard) Course Numbers and Titles CHEM 495: Senior Seminar CHEM 495: Senior Seminar CHEM 495: Senior Seminar CHEM 495: Senior Seminar Please attach syllabi 9