lab 9 - aidanissocoolherro
advertisement

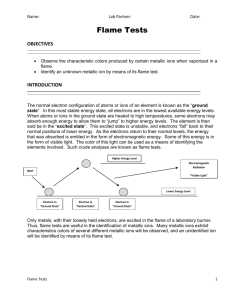
Flame Tests Emily Ma, James Haber Date Completed: November 23rd 2010 Date Due: November 24th 2010 Pre-lab: The normal electron configuration of atoms or ions of an element is known as being in the ground state. In this most stable energy state, all electrons are in the lowest energy levels available. When atoms or ions in the ground state are heated to high temperatures, some electrons may absorb enough energy to allow them to jump to higher energy levels. The element is then said to be in the excited state. This excited configuration is unstable and the electrons fall back to their normal positions of lower energy. As the electrons return to their normal levels, the energy that was absorbed is emitted in the form of electromagnetic energy. Some of this energy may be in the form of visible light. The color of this light can be used as a means of identifying the elements involved. Such crude analyses are known as flame tests. Only metals, with their loosely held electrons, are excited in the flame of a laboratory burner. Thus, flame tests are useful in the identification of metallic ions. Many metallic ions exhibit characteristic colors when vaporized in the burner flame. In this experiment, characteristic colors of several different metallic ions will be observed, and an unidentified ion will be identified by means of its flame test. Purpose/Aim/Objective: The purpose of this lab is to observe the characteristics colors produced by certain metallic ions when vaporized in a flame. Identify an unknown metallic ion by means of its flame test. Equipment/Materials: See Lab Safety: - Always wear goggles during this lab - Wash your hands after the lab - Don’t eat any of the materials - Tie back long hair and tuck in loose clothing - Handle concentrated chemicals with care Procedure: 1. Take out all the needed materials 2. Put on goggles for safety purposes 3. Tie back hair; tuck in clothes if needed 4. Place eight clean tubes into a test tube rack 5. Into the first seven tubes, pour 5 mL of a different nitrate solution; pour 5 mL of the unknown solution into the eighth tube 6. Pour about 10 mL of concentrated hydrochloric acid into a 50 mL beaker 7. Clean the wire loop by dipping the loop into the acid and heating the loop in the outer edge of the burner flame until no color is observed in the flame 8. Dip the clean wire loop into one of the nitrate solutions by placing the loop in the outer edge of the burner flame and moving the loop up and down 9. Note the color of the flame and record it 10. Repeat steps 7,8,9 for the other nitrate solutions and the unknown and record it 11. Clean up after yourself Observations/Data: Metallic Ion Color in Flame Na+ Bright Orange K+ Light pink/ purple Li+ Red Ca2+ Red-orange Sr2+ Red Ba2+ Yellow green Cu2+ Bright green Unknown- K Light purple pink Questions: 1. What inaccuracies may be involved in using flame tests for identification? Inaccuracies may be involved in using flame tests for identification because chemicals could be not cleaned off properly, and end up messing up the identification of another chemical, they could be misinterpreted as different colors by different people, and they are short, so you have to look closely in order to identify colors properly. Also, there are many that emit similar colors of light, so they could be mixed up during identification. 2. Which pairs of ions produce similar colors in the flame tests? K and the unknown, Ba and Cu, and Li and Sr produced similar colors in the flame tests. 3. Explain how the colors observed in the flame tests are produced. When an atom or ion is heated, it moves from a ground state to an excited state. As the atom or ion is now in an unstable electron configuration, the electrons fall back into their normal positions of lower energy known as the ground state. As they fall back to their normal positions, the energy absorbed is emitted in the form of visible light. 4. 5. Define these terms: a) Quanta- the fundamental unit of electromagnetic energy b) Ground state- the normal electron configuration of atoms or ions of an element; the most stable energy state where all electrons in the lowest energy levels are available c) Excited state- when atoms or ions in the ground state are heated to high temperatures and some electrons absorb enough energy to allow them to jump up to higher energy levels; unstable What is a spectroscope? What is observed if the flame tests are viewed through a spectroscope? A spectroscope is an optical device for producing and observing a spectrum of light or radiation from any source. If the flame tests are viewed through a spectroscope, then you can see several bright colored lines on a black background. By comparing the positions of said lines to the known emission spectra of compounds, we can identify the element. Conclusion: This lab allowed us to observe the characteristic colors produced by certain metallic ions when vaporized in a flame. We used a Bunsen burner to heat the metallic ions, and the color of the light observed would be recorded. An atom or ion would be heated to move it from the ground state to the excited state. When said atom or ion moves from the excited state back to the ground state, the energy absorbed initially, is released in the form of light. This color can be used to characterize different elements and help identify unknowns. The unknown chemical in this lab turned out to be a light purple pink. Another element that turned out about the same color was K +, or potassium. Using these tests, we can find out what color of light is produced by different metallic ions and can use the results to identify unknowns. --