Studies selecting outcomes for use in clinical trials of
advertisement
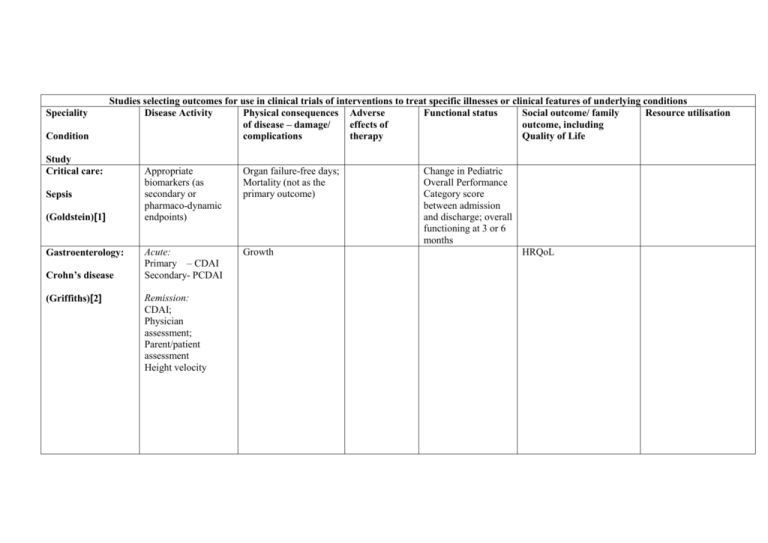
Speciality Condition Studies selecting outcomes for use in clinical trials of interventions to treat specific illnesses or clinical features of underlying conditions Disease Activity Physical consequences Adverse Functional status Social outcome/ family Resource utilisation of disease – damage/ effects of outcome, including complications therapy Quality of Life Study Critical care: Sepsis (Goldstein)[1] Gastroenterology: Crohn’s disease (Griffiths)[2] Appropriate biomarkers (as secondary or pharmaco-dynamic endpoints) Organ failure-free days; Mortality (not as the primary outcome) Acute: Primary – CDAI Secondary- PCDAI Growth Remission: CDAI; Physician assessment; Parent/patient assessment Height velocity Change in Pediatric Overall Performance Category score between admission and discharge; overall functioning at 3 or 6 months HRQoL continued Disease Activity Haematology: Signs-organ specific measures Symptoms-clinican reported Symptoms – patient reported Global ratingphysician Global rating-patient reported GVHD (Pavletic)[3] Neonatology: Neonatal apnoea (Finer)[4] Neonatology: Neonatal cardiovascular instability (Short)[5] Physical consequences of disease – damage/ complications Adverse effects of therapy Functional status Clinician assessed: Grip strength; 2-minute walk time; Karnofsky/ Lansky scale Social outcome/ family outcome, including Quality of Life SF-36 Resource utilisation CHRIS Patient assessed: HAP; ASK; Frequency/ duration/severity of apnoea Associated postnatal morbidity; Measures of organ perfusion Primary outcome: Combined endpoint of mortality or severe neurological outcome Secondary outcomes: NEC;ROP;BPD; Neurodevelopment at 18 months Adverse effects of drugsarrhythmia, hypertension, seizures, hormonal effects Development at 2 years Duration of hospital stay; Duration of assisted ventilation Number of days hospitalized for apnoea continued Disease Activity Neonatology: Acute pain measurements: Behavioural/ Haemodynamic/ Metabolic/ Respiratory/ Renal; Need for supplemental opiates; Frequency of postnatal complications of prematurity Pain (Anand)[6,7] Neonatology Neonatal postoperative cardiac dysfunction (Roth) [8] Endpoint specific to underlying cardiac diagnosis. Secondary measures: blood lactate;oxygenation;c reatinine clearance, BNP, Need for inotropes; cerebral perfusion Physical consequences of disease – damage/ complications Neurobehavioural assessment Adverse effects of therapy Global developmental delay Interventionspecific adverse effects Duration of delayed sternal closure; Mortality beyond 30th postoperative day; Functional status Sleeping pattern; Feeding/Growth; Mother-infant interaction; Cry characteristics; Pain response; Development of addiction; Substance abuse; Anxiety disorders; Neurological/ cognitive development at 18 or 36 months Neurodevelopment at 1 to 2 years Social outcome/ family outcome, including Quality of Life Resource utilisation Length of hospital stay/ ventilation/ NICU stay as represented by physiological definition of criteria to account for inter-centre variations in practice Duration of assisted ventilator; Duration of NICU stay; Duration of hospital stay after cardiac surgery; Disease Activity continued Neonatology: Seizures (Clancy)[9] Neurology: Infantile spasms (West Delphi Group) [10,11] Psychiatry: Bipolar affective disorder (Carlson)[12] Physical consequences of disease – damage/ complications Primary: Cessation of EEG-detected seizures Clinical outcome – spasm cessation Electroclinical outcome- resolution of hypsarrhythmia Relapse-free response; Continued subtle spasm; Time to relapse;Presence of other seizures Primary: Mania (YMS); Depression (CDRS).Secondary:A DHD symptomology; Aggressive behaviour; Global improvement Adverse effects of therapy Functional status Social outcome/ family outcome, including Quality of Life Neurodevelopment at 8 years Development at 2 years;Death and other serious adverse effects associated with the illness; Nonserious adverse events associated with the illness; Presence of and progression to other seizure types Academic outcome and cognitive function Family outcomeCHQ; Other social effects Resource utilisation Disease Activity continued Psychiatry: Seizure frequency Non- epileptic seizures (LaFrance)[13] Respiratory: CF (Ramsey)[14] < 6 years of age Chest x-ray score; Oxygenation; Inflammatory markers; Illness severity; Bronchoscopy/ BAL/brushings >6 years of age As above, and Spirometry; Sputum: microbiology/ DNA Physical consequences of disease – damage/ complications Depression; Personality characteristics; Arousal Growth; Frequency of pulmonary exacerbations Adverse effects of therapy Functional status Social outcome/ family outcome, including Quality of Life HRQoL, including illness perceptions and individual concerns; Employment; Family functioning QWB Resource utilisation ER visits; Hospital admission Disease Activity continued Respiratory: Asthma- Disease damage/ complications Adverse effects of therapy Functional status Social outcome/ family outcome/Quality of Life Symptoms FEV1/FVC PEFR Admission to hospital (Smith)[15] acute chronic Rheumatology: IIM (IMACS)[16–20] Symptoms FEV1/FVC PEFR Frequency of medication use; Bronchial hyperresponsiveness Global activity (physician/parent/ patient assessment); Muscle strenghth (Manual testing); Enzymes: CK, aldose, LD, AST, ALT; CMAS; Extra-skeletal muscle disease Resource utilisation Core set: Global damage assessment; Assessment of different organ systems (VAS/SDI) Extended core set: MRI: muscle fibrosis/ scarring/ atrophy; serum creatinine; cutaneous assessment Functional status HRQoL HAQ/CHAQ HRQoL (SF36) Disease Activity Disease damage/ complications Global assessmentphysician; parent/ child; activity tool; AntiDNA antibody; 24 hr proteinuria Serum creatinine Global assessmentphysician; parent/ child; activity tool; Muscle strength; Laboratory: Muscle enzymes Global damage toolSDI; physician; Height and weight Pubertal stage: Tanners; menses Global assessmentphysician; MDI Height and weight; Pubertal stage: Tanners; menses Muscle strength-CMAS CHAQ Physician global assessment; Number of active joints; ESR Number of joints with limited range of movement Functional ability continued Rheumatology: SLE (PRINTO)[21–23] DM (PRINTO) Rheumatology: Juvenile arthritis (Giannini) [24] Adverse effects of therapy Functional status Social outcome/ family outcome/Quality of Life Resource utilisation Overall well being (Parent/ child reported) Abbreviations: ADHD= Attention Deficit Hyperactivity Disorder; ASK= Activity Scale for Kids; AST= aspartate aminotransferase; ALT= Alanine aminotransferase; BAL= Broncheoalveolar lavage; BNP= Brain Natriuretic Peptide; BPD=Bronchopulmonary Dysplasia; CDAI= Crohns Disease Activity Index; CDRS= Childrens Depression Rating Scale; CHAQ= Childhood Health Assessment Questionnaire; CHQ=Child Health Questionnaire; CHRIS= Child Health Rating Inventories; CK= Creatinine Kinase; CMAS=Childhood Myositis Assessment Scale; ESR= Erytherocyte Sedimentation Rate; FEV1= Forced Expiratory Volume in 1 second; FVC= Forced Vital capacity; HAP= Human Activity Profile; HAQ= Health Assessment Questionnaire; HRQoL=Health Related Quality of Life; LD= Lactate Dehydrogenase; MDI=Myositis Disease Index; NEC=Necrotising Enterocolitis; NICU= Neonatal Intensive Care Unit; PCDAI= Paediatric Crohn’s Disease Index; PEFR= Peak Expiratory Flow Rate; QWB: Quality of Well Being Scale; ROP=Retinopathy of Prematurity; SDI=SLE Collaborating clinic/ACR damage index; VAS= Visual Analogue Scale; YMS=Young mania rating scale Condition Study Dentistry: Dental restoration (De Rouen) [25] Specific clinical trial assessing the safety profile of amalgam in terms of side effects of mercury exposure ( a component of amalgam) Disease Activity Studies selecting outcomes for use in specific clinical trials Consequences of Adverse effects of Functional status Social outcome, family disease – damage/ therapy outcome, including complications Quality of Life Memory; Attention; Motor/ visual skills; IQ; NCV; Clinical signs; Urinary GST/ albumin/ porphyrin Resource utilisation Abbreviations: NCV= Nerve conduction velocity; GST= Glutathione S-Transferase References 1. Goldstein B, Giroir B, Randolph A, Members of the International Consensus Conference on Pediatric Sepsis (2005) International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatric Critical Care Medicine 6: 2-8. 2. Griffiths AM, Otley AR, Hyams J, Quiros AR, Grand RJ, Bousvaros A, Feagan BG, Ferry GR (2005) A review of activity indices and end points for clinical trials in children with Crohn's disease. [Review] [60 refs]. Inflammatory Bowel Diseases 11(2):185-96, 3. Pavletic SZ, Martin P, Lee SJ, Mitchell S, Jacobsohn D et al (2006) Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. Response criteria working group report. Biology of Blood and Marrow Transplantation 12: 252-266. 4. Finer NN, Higgins R, Kattwinkel J, Martin RJ (2006) Summary proceedings from the apnea-of-prematurity group. Pediatrics 117(3 Pt 2):S47-51, 5. Short BL, Van MK, Evans JR (2006) Summary proceedings from the cardiology group on cardiovascular instability in preterm infants. Pediatrics 117: Supplement-9. 6. Anand KJS, Aranda JV, Berde CB, Buckman S, Capparelli EV (2005) Analgesia and anesthesia for neonates: study design and ethical issues. Clinical Therapeutics 27: 814-843. 7. Anand KJ, Aranda JV, Berde CB, Buckman S, Capparelli EV et al (2006) Summary proceedings from the neonatal pain-control group. Pediatrics 117: 8. Roth SJ, Adatia I, Pearson GD, Members of the Cardiology Group (2006) Summary proceedings from the cardiology group on postoperative cardiac dysfunction. Pediatrics 117(3 Pt 2):S40-6, 9. Clancy RR (2006) Summary proceedings from the neurology group on neonatal seizures. Pediatrics 117(3 Pt 2):S23-7, 10. Lux AL, Osborne JP (2004) A proposal for case definitions and outcome measures in studies of infantile spasms and West syndrome: consensus statement of the West Delphi group. [Review] [26 refs]. Epilepsia 45(11):1416-28, 11. Osborne JP, Lux A (2001) Towards an international consensus on definitions and standardised outcome measures for therapeutic trials (and epidemiological studies) in West syndrome. [Review] [7 refs]. Brain & Development 23(7):677-82, 12. Carlson GA, Jensen PS, Findling RL, Meyer RE, Calabrese J et al (2003) Methodological issues and controversies in clinical trials with child and adolescent patients with bipolar disorder: report of a consensus conference. Journal of Child & Adolescent Psychopharmacology 13(1):13-27, 13. LaFrance WC, Jr., Alper K, Babcock D, Barry JJ, Benbadis S, Caplan R, Gates J, Jacobs M, Kanner A, Martin R, Rundhaugen L, Stewart R, Vert C, for the NES Treatment Workshop participants (2006) Nonepileptic seizures treatment workshop summary. Epilepsy & Behavior 8(3):451-61, 14. Ramsey BW, Boat TF (1994) Outcome measures for clinical trials in cystic fibrosis. Summary of a Cystic Fibrosis Foundation consensus conference. [Review] [120 refs]. Journal of Pediatrics 124(2):177-92, 15. Smith MA, Leeder SR, Jalaludin B, Smith WT (1920) The asthma health outcome indicators study. Australian & New Zealand Journal of Public Health 69-75. 16. Miller FW, Rider LG, Chung YL, Cooper R, Danko K et al (2001) Proposed preliminary core set measures for disease outcome assessment in adult and juvenile idiopathic inflammatory myopathies. Rheumatology 40: 1262-1273. 17. Rider LG, Giannini EH, Brunner HI, Ruperto N, James-Newton L, Reed AM, Lachenbruch PA, Miller FW (International consensus on preliminary definitions of improvement in adult and juvenile myositis. Arthritis & Rheumatism 2004 Jul; 50: 2281-2290. 18. Rider LG (2002) Outcome assessment in the adult and juvenile idiopathic inflammatory myopathies. [Review] [213 refs]. Rheumatic Diseases Clinics of North America 28(4):935-77, 19. Rider LG, Giannini EH, Harris-Love M, Joe G, Isenberg D et al (2003) Defining Clinical Improvement in Adult and Juvenile Myositis. [Review] [72 refs]. Journal of Rheumatology 30(3):603-17, 20. Oddis CV (2005) Outcomes and disease activity measures for assessing treatments in the idiopathic inflammatory myopathies. Current rheumatology reports 7: 87-93. 21. Ruperto N, Ravelli A, Cuttica R, Espada G, Ozen Set al (2005) The Pediatric Rheumatology International Trials Organization criteria for the evaluation of response to therapy in juvenile systemic lupus erythematosus: prospective validation of the disease activity core set. Arthritis & Rheumatism 52: 2854-2864. 22. Ruperto N, Ravelli A, Murray KJ, Lovell DJ, Andersson-Gare B (2003) Preliminary core sets of measures for disease activity and damage assessment in juvenile systemic lupus erythematosus and juvenile dermatomyositis. [Review] [47 refs]. Rheumatology 42(12):1452-9, 23. Ruperto N, Ravelli A, Oliveira S, Alessio M, Mihaylova D et al (2006) Pediatric Rheumatology International Trials Organization/American College of Rheumatology provisional criteria for the evaluation of response to therapy in juvenile systemic lupus erythematosus: prospective validation of the definition of improvement. Arthritis & Rheumatism 55(3):355-63, 24. Giannini EH, Ruperto N, Ravelli A, Lovell DJ, Felson DT, Martini A (1997) Preliminary definition of improvement in juvenile arthritis.[see comment]. Arthritis & Rheumatism 40(7):1202-9, 25. DeRouen TA, Leroux BG, Martin MD, Townes BD, Woods JS, Leita?o J, Castro-Caldas A, Braveman N (2002) Issues in design and analysis of a randomized clinical trial to assess the safety of dental amalgam restorations in children. Controlled Clinical Trials 23: 301-320.