Trinity Medical Center Cancer Treatment Protocols
advertisement

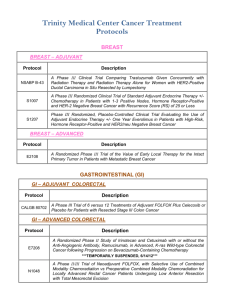
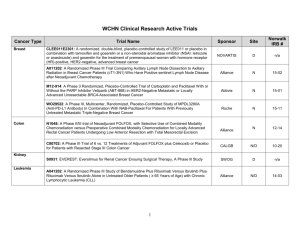
Trinity Medical Center Cancer Treatment Protocols BREAST BREAST – ADJUVANT Protocol Description S1007 A Phase III Randomized Clinical Trial of Standard Adjuvant Endocrine Therapy +/Chemotherapy in Patients with 1-3 Positive Nodes, Hormone Receptor-Positive and HER-2 Negative Breast Cancer with Recurrence Score (RS) of 25 or Less S1207 Phase III Randomized, Placebo-Controlled Clinical Trial Evaluating the Use of Adjuvant Endocrine Therapy +/- One Year Everolimus in Patients with High-Risk, Hormone Receptor-Positive and HER2/neu Negative Breast Cancer BREAST – ADVANCED Protocol E2108 Description A Randomized Phase III Trial of the Value of Early Local Therapy for the Intact Primary Tumor in Patients with Metastatic Breast Cancer GASTROINTESTINAL (GI) GI – ADJUVANT COLORECTAL Protocol CALGB 80702 Description A Phase III Trial of 6 versus 12 Treatments of Adjuvant FOLFOX Plus Celecoxib or Placebo for Patients with Resected Stage III Colon Cancer GI – ADVANCED COLORECTAL Protocol Description E7208 A Randomized Phase II Study of Irinotecan and Cetuximab with or without the Anti-Angiogenic Antibody, Ramucirumab, in Advanced, K-ras Wild-type Colorectal Cancer following Progression on Bevacizumab-Containing Chemotherapy N1048 A Phase II/III Trial of Neoadjuvant FOLFOX, with Selective Use of Combined Modality Chemoradiation vs Preoperative Combined Modality Chemoradiation for Locally Advanced Rectal Cancer Patients Undergoing Low Anterior Resection with Total Mesorectal Excision OTHER GI Protocol CALGB 80803 Description Randomized Phase II Trial of PET Scan-Directed Combined Modality Therapy in Esophageal Cancer ***PENDING*** E2211 A Randomized Phase II Study of Temozolomide or Temozolomide and Capecitabine in Patients with Advanced Neuroendocrine Tumors RTOG 1010 A Phase III Trial Evaluating the Addition of Trastuzumab to Trimodality Treatment of HER2-Overexpressing Esophageal Adenocarcinoma ***PENDING*** S1201 A Randomized Phase II Pilot Study Prospectively Evaluating Treatment for Patients Based on ERCC1 (Excision Repair Cross-Complementing 1) for Advanced / Metastatic Esophageal, Gastric or Gastroesophageal Junction (GEJ) Cancer GENITO-URINARY (GU) Protocol Description A031203 Randomized Phase II Study Comparing Cabozantinib with Commercially-Supplied Sunitinib in Patients with Previously Untreated Locally Advanced or Metastatic Renal Cell Carcinoma RTOG 0534 A Phase III Trial of Short Term Androgen Deprivation with Pelvic Lymph Node or Prostate Bed Only Radiotherapy (SPPORT) in Prostate Cancer Patients with a Rising PSA After Radical Prostatectomy S1216 A Phase III Randomized Trial Comparing Androgen Deprivation Therapy + TAK700 with Androgen Deprivation Therapy + Bicalutamide in Patients with NewlyDiagnosed Metastatic Hormone-Sensitive Prostate Cancer GYNECOLOGIC Protocol GOG 0238 Description A Randomized Trial of Pelvic Irradiation with or without Concurrent Weekly Cisplatin in Patients with Pelvic-Only Recurrence of Carcinoma of the Uterine Corpus HEAD AND NECK Protocol Description E1305 A Phase III Randomized Trial of Chemotherapy with or without Bevacizumab in Patients with Recurrent or Metastatic Head and Neck Cancer E1311 A Randomized, Placebo-Controlled Phase II Trial of Afatinib as Adjuvant Therapy Following Chemoradiation in Patients with Head and Neck Squamous Cell Carcinoma at High Risk of Recurrence HEMATOLOGIC Protocol E1412 Description Randomized Phase II Open Label Study of Lenalidomide R-CHOP (R2CHOP) vs RCHOP (Rituximab, Cyclophosphamide, Doxorubicin, Vincristine and Prednisone) in Patients with Newly-Diagnosed Diffuse Large B Cell Lymphoma ***PENDING*** E1A11 Randomized Phase II Trial of Bortezomib, Lenalidomide and Dexamethasone (VRd) vs Carfilzomib, Lenalidomide and Dexamethasone (CRd) followed by Limited or Indefinite Duration Lenalidomide Maintenance in Patients with NewlyDiagnosed Symptomatic Multiple Myeloma ***PENDING*** S1211 S1304 A Randomized Phase I/II Study of Optimal Induction ***PENDING*** A Phase II Randomized Study Comparing Two Doses of Carfilzomib with Dexamethasone for Multiple Myeloma Patients with Relapsed or Refractory Disease LUNG SMALL CELL LUNG Protocol CALGB 30610 E2511 Description Phase III Comparison of Thoracic Radiotherapy Regimens in Patients with Limited Small Cell Lung Cancer Also Receiving Cisplatin and Etoposide Phase I and Randomized Phase II Double-Blind Clinical Trial of Cisplatin and Etoposide in Combination with Veliparib (ABT-888) or Placebo as Frontline Therapy for Extensive Stage Small Cell Lung Cancer ***PHASE II PENDING*** NON-SMALL CELL LUNG Protocol S0819 Description A Randomized, Phase III Study Comparing Carboplatin/Paclitaxel or Carboplatin / Paclitaxel / Bevacizumab with or without Concurrent Cetuximab in Patients with Advanced Non-Small Cell Lung Cancer ***TEMPORARILY CLOSED, 6/1/14*** MELANOMA Protocol Description A091201 Randomized Phase II Study Comparing the MET Inhibitor Cabozantinib to Temozolomide / Dacarbazine in Ocular Melanoma E2607 Phase II Trial of Dasatinib in Patients with Unresectable Locally Advanced or Stage IV Mucosal, Acral and Solar Melanomas E3611 A Randomized Phase II Study of Ipilimumab at 3 mg/kg or 10 mg/kg Alone or in Combination with High Dose Interferon-α in Advanced Melanoma E3612 A Randomized Phase II Trial of Ipilimumab with or without Bevacizumab in Patients with Unresectable Stage III or Stage IV Melanoma SYMPTOMS CONTROL Protocol A221101 A221102 Description The Use of Armodafinil (Nuvigil®) to Reduce Cancer-Related Fatigue in Patients with Glioblastoma Multiforme: A Randomized, Novel Adaptive Design Study Randomized Double-Blind Placebo-Controlled Study of Subcutaneous Testosterone in the Adjuvant Treatment of Postmenopausal Women with Aromatase Inhibitor-Induced Arthralgias ***TEMPORARILY SUSPENDED, 11/11/14*** R25 CA163197 Psychological Changes in Cancer Patients Participating in a Biobehavioral Intervention: A Program Evaluation