(1) ELECTROCHEMICAL CAPACITORS
advertisement

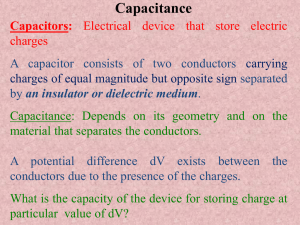
ELECTROCHEMICAL CAPACITORS Their Nature, Function, and Applications By Brian E. Conway Introduction Electrochemical capacitors provide a mode of electrical charge- and energy-storage and delivery, complementary to that by batteries. The capacitance of a capacitor is proportional to the area of the contact plates and the dielectric constant of the medium between the plates, and it is inversely proportional to the separation between the plates. In relation to electrochemical capacitors, to be discussed below, the capacitance of dielectric capacitors is very small being on the order of microfarads or nanofarads for small devices on the order of mm or cm in dimensions. Electrochemical capacitors are a special kind of capacitor based on charging and discharging the interfaces of high specific-area materials such as porous carbon materials or porous oxides of some metals. They can store electric charge and corresponding energy at high densities in an highly reversible way, as does a regular capacitor, and hence can be operated at specific power densities (in watts/kg) substantially higher than can most batteries. Their capacitance for a given size of the device is thus much higher, by a factor of 10,000 or so, than those achievable with regular capacitors. For this reason proprietary names such as "Supercapacitors" or "Ultracapacitors" have been coined to describe their performance. While they function formally like rechargeable batteries in storing or delivering electric charge, their mechanisms of charge storage are quite different, in most cases, from those operating in batteries. Thus, electrochemical capacitors are not substitutes for batteries but rather are to be regarded as complementary to them for charge storage or delivery. They can offer advantageously fast charging or discharging rates over most batteries of comparable volume but their energy density is usually less, by a factor of 3 to 4, than that of batteries. Their high power or power densities, however, enables them to be employed in interesting complementary ways in hybrid systems with batteries. The double-layer capacitance at electrode interfaces An important class of electrochemical capacitors utilizes the so-called double-layer capacitance that arises at all electrode interfaces with electrolyte solutions or ionic melts. The specific capacitances of electrode double layers are very large, some 10,000 times those of ordinary dielectric capacitors, per cm2 area. The reason for this is that the separation of charges in electrochemical double layers is on the order of 0.3-0.5 nanometers (nm = one billionth of a meter) instead of 10 to 100 nm with oxide-film dielectrics (electrolytic capacitors) or 1000 nm with very thin mica or polystyrene dielectric-film hardware capacitors. Hence, it is seen that with large specific-area porous electrodes, for example at carbons having say 1000 m2/g of material and exhibiting, say, 15 µF/(real cm2) of double-layer capacitance in some suitable electrolyte solution, the accessible capacitance "C" is 1000 (m2/g) × 10,000 (cm2/m2) × 15 (µF/cm2) = 150 million µF/g, that is 150 farads/g, a very large capacitance! Hence the term "supercapacitors" or "ultracapacitors" for devices based on double-layer capacitance at high-area substrates. The high degree of reversibility of charge acceptance and delivery, and hence capability for excellent operating power levels compared with batteries of comparable size arises because no slow chemical processes or phase changes take place between charge and discharge as they do in most battery-type electrical charge generating devices. It is the essence of battery-type charge/discharge processes that faradaic processes take place leading to major chemical and structural changes of the electrochemical reactive materials, for example conversions of lead dioxide to lead sulfate and lead metal to lead sulfate in discharge of the lead-acid battery which, as is well known, limits charge/discharge to a cycle life of 1000 to 3000, depending on rates of charge and discharge, and temperature. By contrast, electrochemical double-layer and oxide-type capacitors can exhibit cycle lives up to one million under suitable conditions. This is because only storage and delivery of electrostatic charge takes place at the extended two-dimensional interface of high-area materials and no irreversible or slow chemical phase changes take place as they do between three-dimensional chemical materials in rechargeable batteries. This is a fundamental difference between the electrochemical behavior and properties of electrochemical capacitors relative to those of batteries. It was stated earlier in this article that charging and discharging of electrochemical capacitors has commonly been perceived as a process much more reversible than that for batteries and hence being capable of operation at high power densities. While, in practice, this is largely true, charging of the high-area, porous-electrode structures that are required for achieving large capacitance densities (farads/g or farads/cm3) encounters limitations of rate due to the distributed electrolytic and contact resistances within the pore structure of such materials. In the simplest analysis, any practical capacitor device behaves as if an ohmic resistance is in series with it, the so-called equivalent series resistance. The presence of real or equivalent series resistance in the operating equivalent circuit of any capacitor introduces an ir potential drop in the process of charging or discharging and this drop depends, of course, on the charging rate (current) leading to distortion of the charging curve of accumulated charge against voltage, in time. When the distributed resistance effect also operates, as it normally does, the distortion effect becomes more complex but has been experimentally and computationally evaluated. This situation leads to limitation of the rates of which charging or discharging of porous capacitor electrodes can be conducted and is additional to any equivalent series resistance effects a practical cell may experience due to cell design and electrolyte resistance. It must be stated, however, that in state-of-the-art developments of electrochemical capacitors, using aqueous-solution electrolytes, the above distributed-resistance effect has been substantially minimized so that devices having high operating power have been successfully engineered and marketed. Nevertheless, with non-aqueous electrolyte capacitors, which have higher operating voltages up to 3.0 to 3.5 V (hence 9 to 12 times energy density which depends on the square of maximum operating voltage), the distributed ohmic effects are more significant so that achievable operating power levels are less than those attainable with aqueous electrolyte devices. Electrochemical capacitors based on pseudocapacitance A different kind of capacitance can arise at electrodes of certain kinds, for example ruthenium dioxide, when the extent of faradaically admitted charge depends linearly, or approximately linearly, on the applied voltage. For such a situation, the electrode behavior is equivalent to, and measurable as, a capacitance. This capacitance can be large but it is faradaic and not electrostatic in origin. This is hence an important difference from the nature of double-layer capacitance, so it is called "pseudocapacitance". This kind of pseudocapacitance can originate when an electrochemical charge-transfer process takes place to an extent limited by a finite quantity of reagent or of available surface. Several examples of pseudocapacitance can arise, but the capacitance function is usually not constant and, in fact, is usually appreciably dependent on potential or state of charge. However, when the process is surface limited, and is proceeding in several one-electron stages, a broad range of significant capacitance values arises as is found with ruthenium dioxide electrodes where the pseudocapacitance is almost constant (within 5%) over the full operating voltage range. Some other metal oxides behave similarly but only over smaller operating voltage ranges. The ruthenium dioxide pseudocapacitance provides one of the best examples of electrochemical (pseudo)capacitance as, in addition to the almost constant capacitance over a wide voltage range (over a 1.4 V range), its reversibility is excellent, with a cycle life over several hundred-thousand cycles. Furthermore, the pseudocapacitance can increase the capacitance of an electrochemical capacitor by as much as an order of magnitude over that of the double-layer capacitance. However, its cost prevents its large-scale use so that it has been employed mainly in military applications. General industrial production of electrochemical capacitors follows that of battery cell procedures with automatic production-line machinery. Cell designs are of various kinds including cylindrical, prismatic, button, or coin types, with some larger embodiments being of cake-tin sizes or larger, and some multi-cell series for higher voltage with bipolar electrodes having edge seals. In series configurations for high-voltage applications, balancing of unit cell performance and behavior is a technological challenge. Another application is in electrical research experiments where very high energy and high-rate discharges are required, for example through gases for high-energy spark or arc generation; cold-start assist for diesel locomotives; emergency back-up power for computer systems; stationary power-system load leveling or bridging for short-period power outages; energy source for initial heating of catalytic converter units; energy collection and storage from windmill dynamos. Abstracted from Electrochemistry Encyclopedia (http://electrochem.cwru.edu/ed/encycl/)