MLT 210 Immunology - Moberly Area Community College
advertisement

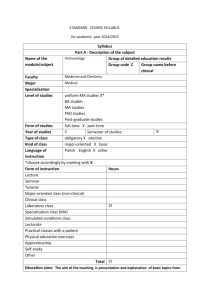
CATALOGUE #MLT 210 CIP #51.1004 DATE: February 1, 2008 Moberly Area Community College COMMON SYLLABUS MLT 210: Immunology Current Term Instructor: Office Number: Office Hours: Contact information: Catalog description: MLT 210 Immunology (w/clinicals) (2–1–3) The course covers concepts and applications of immunology, the immune system, and how to perform and interpret associated tests. The student must satisfactorily perform in a laboratory setting as well as pass written tests. Prerequisite: BOE 171 Medical Terminology, BIO 205 Human Anatomy, BIO 209 Physiology and MLT 150 Laboratory Methods and Management. Text: Rittenhouse, Contemporary Clinical Immunology. 13th Edition, Davis Publishing. ISBN: 978-0-13-510424-8 Polansky, Quick Review Cards For Clinical Lab, Davis FA, ISBN: 978-0-8036-0459-9. Other Required Materials: Handouts, videos, training aids and transparencies as provided by the instructor Purpose of the course: In this course students study the basic concepts of immunology, the theory of clinical assays performed in serology and molecular testing, and disease states involving the immune system. Course content includes basic elements of immune response but also case studies and interpretation of test results. Cognitive Course Objectives: It is the responsibility of the student to learn the following course material presented in Immunology lectures, handouts, textbooks, audiovisuals, and computer-assisted learning: 1. Differentiate the components of the immune system and describe the process of recognition and disposal of foreign material. 2. Describe the cells of the immune system and discuss their interactions. 3. Describe and compare the functions of mediators of the immune system, for example complement, cytokines, and interleukins. 2/5/2016 1 4. Describe major methodology used to diagnose immunological disorders including the following: Agglutination Preceipitation Nephelometry Turbidimetry Electrophoretic techniques Immunoassays (EIA, ELISA, IFA, DFA, RIA) Molecular Methods 5. Discuss specimen collection and handling, test quality and sources of error. 6. Discuss the etiological agents, signs and symptoms, complications of infection, and diagnostic methods for streptococcal infections. 7. State the etiological agent, discuss the signs, symptoms, and progression of infection, and describe testing methods used in the diagnosis of syphilis. 8. Describe the etiological agent, discuss the nature of the infection, and describe the methods used to diagnose Lyme disease. 9. Explain the purpose of the Weil-Felix Test. 10. Discuss the purpose of testing for cold agglutinins and describe the procedure. 11. Describe the etiological agent, discuss the nature of the infection, and explain the methods used to diagnose infection with Toxoplasma gondii. 12. Discuss autoimmune disease states and diagnostic testing used in the clinical laboratory. Psychomotor Course Objectives: Students are responsible for knowing how to do the following to minimal competency standards. In order to develop skills to this level, they will receive instruction and practice in the student lab and at the affiliate site. 1. Identify correct specimen collection for specified immunological determinations and correctly process specimens for testing. 2. Follow laboratory policies for record keeping and reporting. 3. Follow written procedures. 4. Evaluate specimen quality and describe corrective action to solve problems with it. 5. Safely handle and dispose of infectious materials. 6. Utilize quality controls in immunological procedures; evaluate performance from QC results, and initiate corrective action if quality control fails. 7. Perform daily maintenance routines as directed by mentors. 8. Correctly perform specified immunological testing such as agglutination, precipitation, EIA, etc. and interpret results. 9. Discuss the test principles, sources of error, expected values, biological false positives or negatives, and clinical significance of immunological procedures not performed in the laboratory such as FTA-AB, C3, C4, Toxoplasma antibody, and Lyme Disease antibody. 10. Troubleshoot procedures and take appropriate action as needed. Affective Course Objectives: At the end of the course the student will be able to display for following behaviors and attitudes: 2/5/2016 2 1. 2. 3. 4. 5. 6. Demonstrate responsibility toward patients and colleagues. Maintain confidentiality. Accept instruction and constructive criticism. Demonstrate initiative and resourcefulness. Exhibit the ability to work independently. Demonstrates attention to detail and quality Course Content: I. Definition of immunity A. First line of defense B. Natural immunity 1. Cellular a. Neutrophils b. Eosinophils c. Basophils d. Mast cells e. Monocytes f. Tissue macrophages g. Dendritic cells h. Phagocytosis 2. Humoral a. C-reactive protein b. Serum amyloid A c. Complement d. Mannose-binding protein e. Alpha-1 antitrypsin f. Haptoglobin g. Fibrinogen h. Ceruloplasmin C. Adaptive immunity 1. Cellular 2. Humoral II. Inflammatory process III. Antigen characteristics A. General characteristics B. Histocompatibility antigens C. Autoantigens D. Blood group antigens E. Chemical nature of antigens F. Physical nature of antigens IV. Antibody characteristics A. Immunoglobin classes B. Antibody structure 1. Fab, Fc, and hinge molecular components 2. IgM 3. Immunoglobulin variants 2/5/2016 3 V. VI. VII. VIII. IX. C. Antibody synthesis 1. Primary response 2. Secondary response D. Functions E. Immune complexes F. Monoclonal antibody Lymphoid System A. Primary B. Secondary C. Surface marker on lymphocytes D. B cells E. T cells Cytokines A. Interferons B. Tumor necrosis factor C. Interleukins D. Chemokines E. Interferons Complement A. Classical pathway B. Alternative pathway C. Lectin pathway D. System controls 1. Fluid phase regulators 2. Cell-bound regulators E. Receptors that amplify the immune response F. Disease states G. Laboratory assays and findings Safety in the Immunology lab Laboratory methods A. Precipitation B. Radial Immunodiffusion C. Immunoelectrophoresis D. Immunofixation electrophoresis E. Agglutination 1. Direct agglutination 2. Passive agglutination 3. Reverse passive agglutination 4. Agglutination inhibition F. Labeled immunoassays 1. RIA 2. EIA 3. IFA 4. FPIA G. RAST H. Molecular Methods 2/5/2016 4 X. XI. XII. XIII. XIV. XV. 1. Nucleic acid structure and organization 2. Nucleotide structure and sequence 3. DNA and RNA molecular structure and function a. Genes and genetic code b. Chromosomes c. Nonchromosomal elements of a genome d. Replication 4. Nucleic acid-based methods a. Nucleic acid hybridization methods b. Solid support (1) Filter (2) Southern (3) Sandwich 5. Amplification a. PCR Streptococcal infections A. Gp A infection characteristics B. Antibody detection Spirochete diseases A. Syphilis 1. Characteristics of the organism 2. Stages of disease 3. Laboratory diagnosis B. Lyme disease 1. Characteristics of the organism 2. Stages of disease 3. Laboratory diagnosis Rickettsia Cold Agglutinin Toxoplasmosis Autoimmunity A. Hypersensitivity B. Diseases of autoimmunity 1. Lupus erythematosis a. Clinical signs b. Immunologic findings c. Laboratory diagnosis 2. Rheumatoid arthritis a. Clinical signs b. Immunologic findings c. Laboratory diagnosis 3. Hashimoto’s thyroididis 4. Grave’s disease 5. Type I diabetes mellitus 6. Multiple Sclerosis 7. Myasthenia gravis 2/5/2016 5 8. Goodpasture’s syndrome Student Laboratory/Clinical Schedule: Students will spend some student laboratory time learning how to perform selected Immunology/Serology tests. Because in most laboratories serology testing is performed in hematology, chemistry, and/or microbiology, students are advised to document laboratory test experience wherever they acquire it. The following may be performed in Student Laboratory Serial dilution Latex agglutination: CRP Flocculation: RPR Radial Immunodiffusion Enzyme Immunoassay: HCG Streptozyme or ASO Infectious mononucleosis testing Protein Electrophoresis Modified Westergren (ESR) Rheumatois arthritis General Notes: Library assignments, periodicals, computerized modules, and guest speakers may be used as appropriate. Assessment of Student Learning: In both the didactic and the laboratory portions of the course, the student must achieve 78% or greater. Failure to achieve this minimum score will result in dismissal from the program. In the laboratory portion of the course, the final grade will be recorded as “Pass” or “Fail” and registered with the didactic portion. The following grading scale applies to all programs within the Allied Health Division: 100 – 92% = A 83 – 91% = B 78 – 82% = C 66 – 77% = D 65% and below = F 2/5/2016 6 In the lecture portion of the clinical course, the final grade is derived from student performance on examination(s) and/or assignments. Grading/Student Assessment of lecture (didactic) portion of the course: Final grade will be composed of: 1. Unit tests averaged = 60% 2. Case studies, study questions, write-ups, or quizzes averaged = 10% 3. Final exam = 30% TOTAL 100% Grading/Student Assessment of laboratory (student laboratory and clinicals) portion of the course: 1. Skills Checklist: Passing is 78% of the total points available 2. Professional Behaviors Evaluation: Passing is no less than 22 points or 78% Final Pass/Fail clinical grade is the average of the Skills Checklist and the Professional Behaviors Evaluation Statement to Connect Course with General Education Outcomes or Technical Program Outcome Statement: In compliance with MACC’S General Education outcomes, the student who successfully completes this course will be able to: I. Demonstrate effective written and oral communication skills. II. Demonstrate an understanding of scientific principles and computational skills and how to use them to solve problems and make informed decisions. Program Outcomes and Assessments: The Allied Health Department continually strives to meet the needs of the Medical Laboratory Technician student through program improvements. This is a cooperative effort that includes input from the faculty, student, Medical Laboratory Technician Advisory Board, and other appropriate agencies or entities. Students are assessed on mastery of the course concepts and essential skills throughout the courses of the Medical Laboratory Technician Program. Other program assessments include clinical performance criteria, essential skills mastery, the clinical process evaluation, ASCP examination scores, placement rates, and follow-up surveys. 2/5/2016 7 Instructor Policies/Expectations: Instructors of this program expect the following from students: 1. Come to class prepared to discuss or apply important concepts by having read the assigned material or reviewed materials for instrument operation. 2. Participate in class by listening, taking notes, and making contributions to discussions. 3. Consult with faculty for clarification of difficult material or additional resources to consult. 4. Respect the learning environment by averting distractions and disturbances such as ringing cell phones and extraneous conversation in class. 5. Treat instructors and fellow students with consideration, concern, and fairness. 6. Refrain from using a laptop computer during lecture. Academic Dishonesty: MACC board policy is as follows: “Academic dishonesty by students damages institutional credibility and unfairly jeopardizes honest students; therefore, it will not be tolerated in any form.” Forms of academic dishonesty include but are not limited to the following: violations of copyright law, plagiarism, fabrication, cheating, collusion, and other academic misconduct. Incidents of dishonesty regarding assignments, examinations, classroom/laboratory activities, and/or the submission of misleading or false information to the College will be treated seriously. The procedure for handling academic dishonesty is outlined in the Student Handbook (Policy Handbook M.010). In cases of alleged academic dishonesty, the burden of proof is on the student, not on the instructor. Attendance: Students are expected to prepare for and attend all classes and clinical practice. Regular attendance improves probability for success in the program. Habitual tardiness and frequent absences are disruptive to the classroom and cause an unsafe environment in the student laboratory. Instructors carefully plan learning experiences, so it is important as a matter of courtesy and fairness to the class that all individuals be present. Students absent for reasons beyond their control, such as verified personal illness or family illness and/or death, can make up class work. If a student misses so many classes due to extenuating circumstances that the instructor feels the student cannot catch up, the MLT Program Coordinator will send a written report to the Director of Allied Health. Students who miss two consecutive weeks of class during a regular sixteen-week semester or the equivalent ratio of class time during a shorter session will be dropped from that class unless acceptable justification is supplied to the instructor and the Dean of Student Services. Additionally, students who miss more than one-fourth of the class meetings during any scheduled session may be dropped from that class by the instructor if, in the opinion of the instructor, the student does not have a reasonable opportunity to succeed in the class. Tardiness, make-up, and late work: Tardiness to class and clinicals is disruptive and inconsiderate of others. Being on time is mandatory. 2/5/2016 8 Make-up and late work: See the MLT Student Handbook for guidelines. Remember that communication, accountability and responsibility are very important professional behaviors. Refer to the MLT handbook for the following policies: Drop policy Drug/alcohol policy Grade appeal procedure Student code of conduct Student due process and grievance procedure Student rights and privacy act Use of computing resources ADA Statement Students who have disabilities that qualify under the Americans with Disabilities Act may register for assistance through the Office of Access and ADA Services. Students are invited to contact the Access Office to confidentially discuss disability information, academic accommodations, appropriate documentation and procedures. For more information, please call either the Moberly office at (660) 263-4100 x 11240 or the Columbia office at (573) 234-1067 x 12120, or visit our web page at http://www.macc.edu/index.php/services/access-office. 2/5/2016 9