7th Biometrics Europe Meeting - American Statistical Association
advertisement
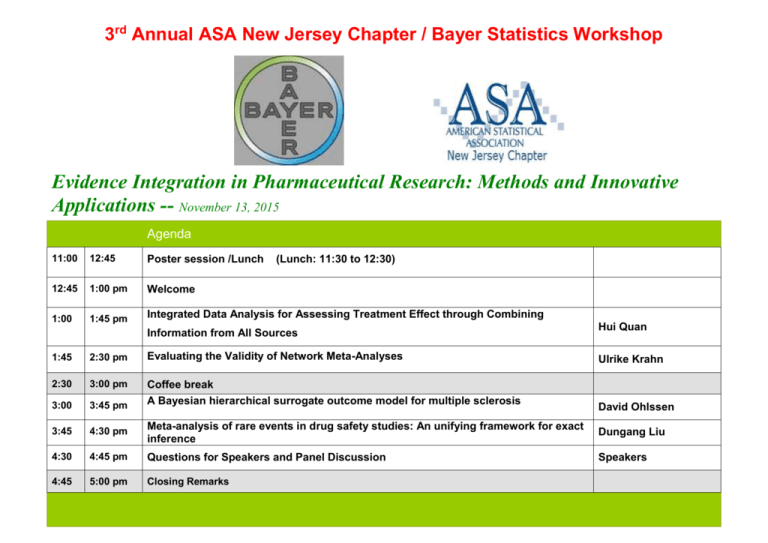
3rd Annual ASA New Jersey Chapter / Bayer Statistics Workshop Evidence Integration in Pharmaceutical Research: Methods and Innovative Applications -- November 13, 2015 Agenda 11:00 12:45 Poster session /Lunch 12:45 1:00 pm Welcome 1:00 1:45 pm Integrated Data Analysis for Assessing Treatment Effect through Combining (Lunch: 11:30 to 12:30) Information from All Sources Hui Quan 1:45 2:30 pm Evaluating the Validity of Network Meta-Analyses 2:30 3:00 pm 3:00 3:45 pm Coffee break A Bayesian hierarchical surrogate outcome model for multiple sclerosis 3:45 4:30 pm Meta-analysis of rare events in drug safety studies: An unifying framework for exact inference Dungang Liu 4:30 4:45 pm Questions for Speakers and Panel Discussion Speakers 4:45 5:00 pm Closing Remarks Ulrike Krahn David Ohlssen Abstract Integrated Data Analysis for Assessing Treatment Effect through Combining Information from All Sources. Hui Quan, Bingzhi Zhang, Christy Chuang-Stein, Byron Jones and some others in the EFSPI IDA efficacy Working Group It is always critical to use a precise estimate of treatment effect for making a claim, evaluating benefit/risk or designing a new study. Utilization of data from all sources in an integrated data analysis/meta-analysis will help us to obtain such a more precise estimate. Depending on the data sources and objectives, there are many approaches for the integrated analysis. These include network metaanalysis, multivariate meta-analysis, model-based meta-analysis as well as methods of borrowing historical data. In this manuscript, we discuss these methods with additional details for implementation and interpretation. We also consider information adaptive repeated cumulative meta-analyses including the corresponding conditional power calculation for making a claim. We apply the integrated analysis with three approaches of taking into account the variability of the overall estimate of treatment effect to sample size calculation for a new trial. Some computation and simulation results are provided. Evaluating the Validity of Network Meta-Analyses Ulrike Krahn, Harald Binder, Jochem König In network meta-analyses, competing treatments are compared simultaneously by pooling the evidence from trials considering different randomized comparisons. The resulting trial network allows estimating the relative effects of all pairs of treatments taking indirect evidence into account. While indirect conclusions are based on the assumption of a consistent network of treatment effects, there may be some direct comparisons that lead to inconsistency and may have side effects on the network estimates of other pairwise comparisons. We have investigated how such direct comparisons can be localized and have developed two graphical approaches for assessing inconsistency in network meta-analyses: For the first graphical approach, the net heat plot, a set of models is explored that results from successively relaxing the assumption of consistency. The change in inconsistency can then be assessed by chi-square statistics and is visualized in detail in the net heat plot. For rendering transparent which direct comparison might have introduced inconsistency in the network, also the contribution of each direct estimate to the network estimates is displayed [1, 2]. For the second graphical approach, we showed that network estimates for single pairwise treatment comparisons can be approximated by the evidence of a subnet that is decomposable into independent paths. Path-based estimates and the estimate of the residual evidence can be used with their contribution to the network estimate to set up a forest plot for the consistency assessment [3]. For supporting the evaluation of validity, we propose in addition a display of the flow of evidence. We have shown that the flow of evidence for a single pairwise network estimate can be displayed by a weighted directed acyclic graph and we have introduced measures to characterize it [4]. All the proposed approaches are illustrated and discussed by two network meta-analyses examples. A Bayesian hierarchical surrogate outcome model for multiple sclerosis David Ohlssen The development of novel therapies in multiple sclerosis (MS) is one area where a range of surrogate outcomes are used in various stages of clinical research. While the aim of treatments in MS is to prevent disability, a clinical trial for evaluating a drugs effect on disability progression would require a large sample of patients with many years of follow-up. The early stage of MS is characterized by relapses. To reduce study size and duration, clinical relapses are accepted as primary endpoints in phase III trials. For phase II studies, the primary outcomes are typically lesion counts based on Magnetic Resonance Imaging (MRI), as these are considerably more sensitive than clinical measures for detecting MS activity. Recently, Sormani and colleagues in “Surrogate endpoints for EDSS worsening in multiple sclerosis” provided a systematic review, and meta-analysis using weighted regression to examine the role of either MRI lesions or relapses as trial level surrogate outcomes for disability. We build on this work by developing a Bayesian three-level model, accommodating the two surrogates and the disability endpoint, and properly taking into account that treatment effects are estimated with errors. Specifically, a combination of treatment effects based on MRI lesion count outcomes and clinical relapse were used to develop a study level surrogate outcome model for the corresponding treatment effects based on disability progression. While the primary aim for developing this model was to support decision making in drug development, the proposed model may also be considered for future validation. Meta-analysis of rare events in drug safety studies: An unifying framework for exact inference Dungang Liu For drug safety analysis in clinical trials where adverse events are often rarely observed, a challenging but recurrent problem is the present of the so-called zero total event studies where both treatment and control groups observe zero events. In this situation, conventional meta-analysis approaches either exclude such studies from the analysis, or apply 0.5 continuity corrections to zero events. Both practices, however, are known to have undesirable consequences in inference. Motivated by this problem, we propose to combine p-value functions (also known as significance functions) associated with the exact tests from individual studies. This approach can incorporate all available data in the analysis without using artificial corrections for zero events. We further show that the idea of combining p-value functions yields an unifying framework for exact meta-analysis, as it permits broad choices for the combining elements, such as tests used in individual studies, and any parameter of interest. We theoretically show that our approach yields statements that explicitly account for the impact of individual studies on the overall inference in terms of efficiency/power and the type I error rate. We demonstrate, through numerically studies in some rare events settings, that our exact approach is efficient and outperforms existing commonly used meta-analysis methods. Speaker Biographies Dr. Hui Quan is currently an associate VP at the Biostatistics and Programming Department of Sanofi. He is the global head of the methodology group. He obtained his PhD degree in statistics from Columbia University in 1990. He has 24 years of pharmaceutical industry experience, 82 publications including 59 statistical papers in peer reviewed statistical journals. He was elected as an ASA fellow in 2008. Dr. Ulrike Krahn works as an Associate Director at Bayer HealthCare, Clinical Statistics Europe. During the past six years before her career at Bayer HealthCare, she worked as biostatistician at university hospitals in Germany (Johannes Gutenberg University Mainz and University Duisburg-Essen). She conducted methodological research and wrote her doctoral thesis in the very active field of “network meta-analyses”. In 2014, her work was acknowledged by the Gustav-Adolf-Lienert-Award of the German Region of the International Biometric Society (IBS-DR). Dr. David Ohlssen is currently a Biometrical Fellow and Advanced Exploratory Analytics team lead, within the Novartis statistical methodology and consulting group, based in East Hanover New Jersey. Since joining Novartis in 2007, he has developed a broad range of experience in applying novel statistical approaches within a drug development setting. Previously, after completing his PhD in Biostatistics at the University of Cambridge, he worked as a research fellow at the MRC Biostatistics Unit (Cambridge UK), where his interests included: diagnostics for Bayesian models, novel clinical trial design and statistical methods for the profiling of health-care providers. His professional activities include serving as a member of the Bayesian DIA Working Group and within the group acting as the chair of the safety meta-analysis sub-team. Dr. Dungang Liu is Assistant Professor of Business Analytics at the University of Cincinnati Lindner College of Business. Prior to joining the Lindner College of Business, Dr. Liu obtained his Ph.D. degree in statistics from Rutgers University and had two-year postdoctoral experience in biostatistics at Yale University. Dr. Liu's research interests include meta-analysis, confidence distribution inference and ordinal data analysis. His research outcomes have been published in leading statistical journals, such as the Journal of American Statistical Association. Driven by his strong interest in statistical application in business and industry, Dr. Liu has been actively involved in consulting projects for health care, aviation and insurance companies.