BMC Oral Health MS: 1472476760174189 Date: 21 November 2015
advertisement
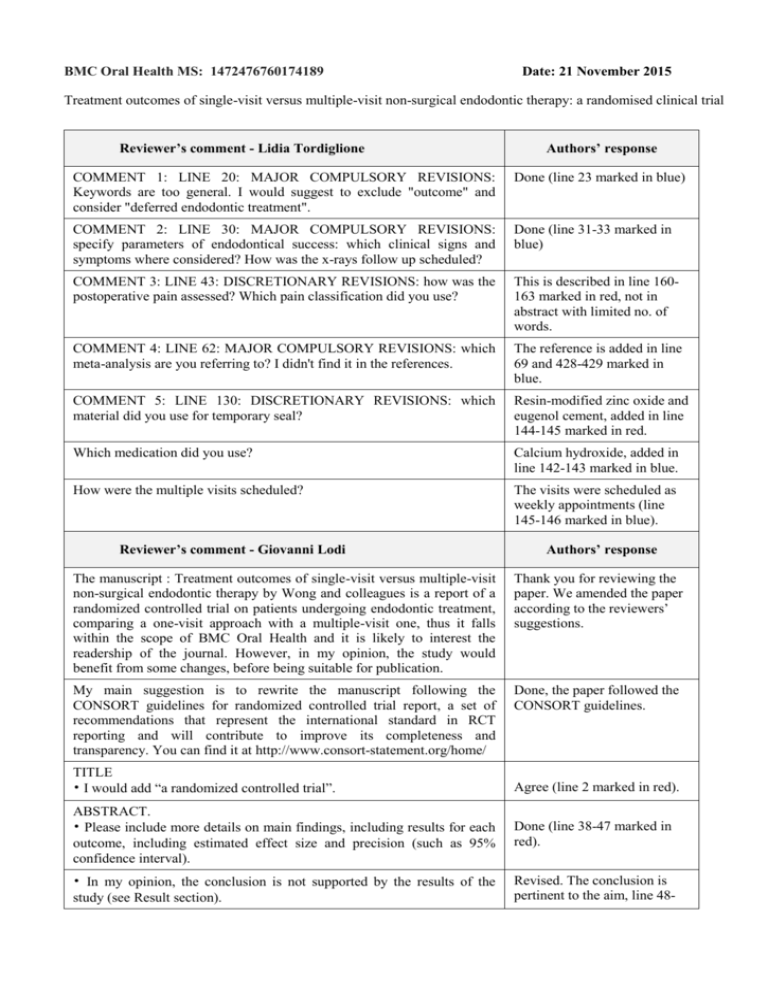
BMC Oral Health MS: 1472476760174189 Date: 21 November 2015 Treatment outcomes of single-visit versus multiple-visit non-surgical endodontic therapy: a randomised clinical trial Reviewer’s comment - Lidia Tordiglione Authors’ response COMMENT 1: LINE 20: MAJOR COMPULSORY REVISIONS: Keywords are too general. I would suggest to exclude "outcome" and consider "deferred endodontic treatment". Done (line 23 marked in blue) COMMENT 2: LINE 30: MAJOR COMPULSORY REVISIONS: specify parameters of endodontical success: which clinical signs and symptoms where considered? How was the x-rays follow up scheduled? Done (line 31-33 marked in blue) COMMENT 3: LINE 43: DISCRETIONARY REVISIONS: how was the postoperative pain assessed? Which pain classification did you use? This is described in line 160163 marked in red, not in abstract with limited no. of words. COMMENT 4: LINE 62: MAJOR COMPULSORY REVISIONS: which meta-analysis are you referring to? I didn't find it in the references. The reference is added in line 69 and 428-429 marked in blue. COMMENT 5: LINE 130: DISCRETIONARY REVISIONS: which material did you use for temporary seal? Resin-modified zinc oxide and eugenol cement, added in line 144-145 marked in red. Which medication did you use? Calcium hydroxide, added in line 142-143 marked in blue. How were the multiple visits scheduled? The visits were scheduled as weekly appointments (line 145-146 marked in blue). Reviewer’s comment - Giovanni Lodi Authors’ response The manuscript : Treatment outcomes of single-visit versus multiple-visit non-surgical endodontic therapy by Wong and colleagues is a report of a randomized controlled trial on patients undergoing endodontic treatment, comparing a one-visit approach with a multiple-visit one, thus it falls within the scope of BMC Oral Health and it is likely to interest the readership of the journal. However, in my opinion, the study would benefit from some changes, before being suitable for publication. Thank you for reviewing the paper. We amended the paper according to the reviewers’ suggestions. My main suggestion is to rewrite the manuscript following the CONSORT guidelines for randomized controlled trial report, a set of recommendations that represent the international standard in RCT reporting and will contribute to improve its completeness and transparency. You can find it at http://www.consort-statement.org/home/ Done, the paper followed the CONSORT guidelines. TITLE • I would add “a randomized controlled trial”. Agree (line 2 marked in red). ABSTRACT. • Please include more details on main findings, including results for each outcome, including estimated effect size and precision (such as 95% confidence interval). Done (line 38-47 marked in red). • In my opinion, the conclusion is not supported by the results of the study (see Result section). Revised. The conclusion is pertinent to the aim, line 48- 50 marked in blue. MATERIALS AND METHODS. • Provide more details on random sequence generation and allocation concealment methods (see CONSORT). Done (line 117-122 marked in red and blue). • Patients recruitment. Please provide clearer inclusion/exclusion criteria. For instance, it is not clear to me whether vital teeth in need of endodontic treatment were included or not. The criteria are provided in line 105-114 marked in red and in Figure 1. In addition, what happened with patients who needed more than one endodontic treatment? Were they randomized more than once? Details added, lined in 119121 marked in blue). • Clinical procedure. Were multiple-visit treatments always two-visit treatments. If not, under which circumstances it was decided for a longer treatment? Multiple-visit treatments could be 2- or 3-visit, the details are added in line 145147 marked in red. • How the duration of the intervention was measured? Authors should state starting and ending points. Added in line 103-104 marked in blue. • What (if any) medication (FANS, antibiotics) was recommended following treatment and in which circumstances? Added in line 149-151 marked in red). • Evaluation. This section is particularly confused. It appears that the first data were collected after six months. If this is true, it makes sense for the radiological outcome, but it does not for clinical outcomes, and for pain in particular. Didn’t Authors recorded pain immediately (6, 12, 24, 48 h) and in the first week following treatment? What do the Author mean by “incidence of pain” (line 177). Details are added in the evaluation, marked in red (line 156 and line 160-163 marked in red). RESULTS • How many patients were enrolled? Added in line 225-226 marked in red and in Figure 1. • Please specify reasons of patient lost at follow-up (line 182). Added in line 228-229 marked in red and in Figure 1. • Please provide information on time when the outcome (success, pain) was recorded. Done, line 227-228 and 249254 marked in blue. • The methods of the reliability were not reported in the M&M section. Added in line 232-234 marked in red. • Tooth lost is a very important outcome, why it was not included among the outcomes of the study in the M&M section. Discussed in line 180-181 and 311-314 marked in red. • How did Authors explain the difference in gender distribution between groups? Is it a possible sign of some unknown bias? This is discussed in line 278280 marked in red. DISCUSSION. • Too long. Make it shorter. Done (no. of word reduced from 1,577 to 1,459).