Heartlands leaflet - Good Hope Eye Clinic
advertisement

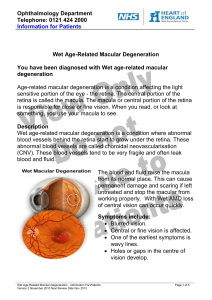
Ophthalmology Department Telephone: 0121 424 2000 Information for Patients Wet Age-Related Macular Degeneration Description Wet age-related macular degeneration consists of leakage into the retina. The leakage derives from abnormal blood vessels, which come from behind the retina - the choroid. The abnormal blood vessels are called choroidal neovascularisation. The choroidal neovascularisation develops, leaks, damages the retina and then scar tissue develops. There are different stages of the disease. Symptoms Symptoms include: blurred vision. Central or fine vision is affected. One of the earliest symptoms is wavy lines. Holes or gaps in the centre of vision develop. Diagnosis The diagnosis is made by a complete ocular examination. This is followed by a fluorescein angiogram. A fluorescein angiogram consists of a dye injection into a vein in the arm, followed by photographs of the eye. The procedure takes approximately ten minutes. Other tests include ocular coherence tomography, a type of ultrasound to assess the amount and distribution of fluid in the retina. Treatment The treatment for wet age-related macular degeneration includes, laser photocoagulation, Triamcinolone, photodynamic therapy, Lucentis, Avastin, Macugen, or a combination of photodynamic therapy and Lucentis. Treatment in wet macular degeneration is Wet Age-Related Macular Degeneration - Information For Patients Version 1 March 2009 Next Review Date Nov 2010 Page 1 of 6 Ophthalmology Department Telephone: 0121 424 2000 Information for Patients aimed at sealing or stopping the leakage, getting rid of the choroidal neovascularisation and preventing further retinal damage. The earlier that treatment is performed, the greater the likelihood of success. When the retinal damage has progressed to scar tissue formation, treatment is unlikely to help. Of all those who have wet macular degeneration, 5-10% are suitable for laser treatment. Laser treatment is a form of welding; the laser destroys the abnormal vessels. Laser treatment is used if the abnormal vessels are away from the centre of vision. It cannot be used if the blood vessels are underneath the centre of vision. For the vast majority of people, laser is not suitable as the abnormal blood vessels are underneath the centre of vision. Photodynamic therapy has been available for some years. It was the first effective treatment for wet macular degeneration, in those where the leakage was underneath the centre of vision. The treatment consists of an injection of dye, followed by application of a low energy laser. Photodynamic therapy destroys the new blood vessels. Photodynamic therapy reduces the extent and severity of visual loss. Vision is still lost with treatment. The amount of vision lost, is less with treatment, than without treatment. Photodynamic therapy is suitable for perhaps 60% of patients with wet macular degeneration. Some types of choroidal neovascularisation do not respond to photodynamic therapy. VEGF stands for vascular endothelial growth factor. Lucentis is a an agent that blocks VEFG. It is given via an injection into the white of eye. VEFG blocking drugs stop the growth of choroidal neovascularisation. Treatment is required on monthly occasions. The trials have shown that more than 30-40% of patients having treatment with Lucentis improve. This means with treatment there is a good chance that your vision will improve. Lucentis is the first and only drug that demonstrates this improvement in this percentage of patients. 90% of all patients treated remained stable or improved their vision. (‘Remained stable’ has been defined in many trials as having the same vision or loss of up to three lines of letters). Patients with all forms of choroidal neovascularisation Wet Age-Related Macular Degeneration - Information For Patients Version 1 March 2009 Next Review Date Nov 2010 Page 2 of 6 Ophthalmology Department Telephone: 0121 424 2000 Information for Patients respond to the treatment. All people with wet macular degeneration can potentially benefit from treatment with Lucentis. Macugen is a VEGF antagonist. It has been shown to be of benefit in patients with all types of choroidal neovascularisation. The effects on vision are as effective as photodynamic therapy. It has not been shown to improve vision to the extent that Lucentis does. Triamcinolone is a steroid type of drug and can in conjunction with photodynamic therapy help in the treatment of choroidal neovascularisation. Long Term Outlook Wet age-related macular degeneration will progress in the absence of treatment with serious loss of central vision. Peripheral or out and around vision will be preserved. I have age-related macular degeneration, what now? The diagnosis needs to be confirmed. This means a complete eye examination, measurement of vision, examination of retina ( back of the eye) and fluorescein angiogram (dye test) and ocular coherence tomography ( type of imaging). The decision on the type of treatment needed is made on the basis of all the above. The important issues are the vision, the type of choroidal neovascularisation and the presence or absence of scarring. Why can’t I have treatment? If your vision is below a certain level, treatment is unlikely to help. The retina has no powers of recovery. If the retina is badly damaged by bleeding or scarring, then treatment is not going to change the long term outlook and is of no benefit. If your disease has been present for some time, scarring may well have developed. Lucentis does not work very well in the presence of scarring. Wet Age-Related Macular Degeneration - Information For Patients Version 1 March 2009 Next Review Date Nov 2010 Page 3 of 6 Ophthalmology Department Telephone: 0121 424 2000 Information for Patients I am going to have Lucentis, what does this mean? Lucentis is of proven benefit in those who have choroidal neovascularisation. It is given via an injection into white of the eye. The injection is given every month (for 3 months). The injection is performed with the aid of local anaesthetic, using an operating microscope. It is performed either in the treatment room of the out patients or in the operating theatre. The injection is not painful and takes no more than a few minutes. After the injection, your vision will be blurred and your eye may be slightly sore. These effects will soon disappear. What is the risk of Lucentis? The administration involves an injection into the eye. Minor problems such as redness, mild soreness and blurred vision are usually temporary. High pressure may develop after the injection. Any injection into the eye can be complicated by infection. If you develop an infection in your eye, complete loss of vision can occur. Early treatment of infection can improve the outlook. If your eye becomes progressively red or sore after the injection, you need to contact the eye clinic (phone numbers at the end). In the long term, repeated ocular injections may predispose to the development of cataracts. What do I do after the Lucentis treatment? You should not rub your eye. You should avoid immersing your eye for 48 hours. You should avoid vigorous exercise for 48 hours, thereafter; you can resume your normal life. If your Lucentis has been combined with photodynamic therapy, you need to avoid bright sunlight for 48 hours. If you are outdoors, you need to wear protective clothing during that time. Am I going to have photodynamic therapy with Lucentis? The trials evaluating Lucentis used a regime of monthly injections for 24 months. These results showed 30-40% of people improved or were stable. There are newer trials looking at the different doses of Wet Age-Related Macular Degeneration - Information For Patients Version 1 March 2009 Next Review Date Nov 2010 Page 4 of 6 Ophthalmology Department Telephone: 0121 424 2000 Information for Patients Lucentis, the timing of treatment and combination therapy with photodynamic therapy. The trials are still underway and firm evidence on this regime is not yet available. You may or may not have Lucentis combined with photodynamic therapy your Ophthalmologist will advise you. Is this experimental? No. Lucentis is of proven benefit in patients with age-related macular degeneration with choroidal neovascularisation. Lucentis was licensed in Europe in February 2006. Treatment regime The optimum treatment regime dose of Lucentis, dosing regime and usage with photodynamic therapy is not yet certain. It is recommended that you have 3 doses Lucentis one per month for 3 months. After your third dose of Lucentis, you will be reviewed monthly with ocular coherence tomography (imaging). Initially you will be reviewed monthly, but the interval may change depending on your progress. On average, patients require 8 treatments with lucentis in the first year and 6 in the second year. The initial use of photodynamic therapy may reduce the need for additional treatment with lucentis. A decision on your further treatment will depend on your response. Lucentis treatment is required until the leakage ceases. Occasionally the leakage can stop and some months later recur. This is the reason that you require follow up with ocular coherence tomography for 24 months following your first treatment. You can consider the initial three injections as an induction regime and the remainder of the injections as maintenance treatment, required when there is disease activity. Wet Age-Related Macular Degeneration - Information For Patients Version 1 March 2009 Next Review Date Nov 2010 Page 5 of 6 Ophthalmology Department Telephone: 0121 424 2000 Information for Patients Contact Us: Emergency 0121 – 424 - 2000 ask for Bleep 2489 (macula nurse) Eye clinic Solihull 0121 – 424 – 4094 Eye clinic Heartlands 0121 – 424 – 40543 Eye clinic Good Hope 0121 – 424 - 9608 Eye Casualty after 5pm and weekends 0121 507 6780 Our commitment to confidentiality We keep personal and clinical information about you to ensure you receive appropriate care and treatment. Everyone working in the NHS has a legal duty to keep information about you confidential. We will always ask you for your consent if we need to use information that identifies you. We will share information with other parts of the NHS to support your healthcare needs, and we will inform your GP of your progress unless you ask us not to. You can help us by pointing out any information in your records which is wrong or needs updating. Additional Sources of Information: You may want to visit our Health Information Centres located at the Main Entrance at Birmingham Heartlands Hospital, Tel: 0121 424 2280, or at the Treatment Centre at Good Hope Hospital Tel: 0121 424 9946 or Email: healthinfo.centre@heartofengland.nhs.uk NHS Direct Telephone 0845 4647 or visit them on the Internet at http://www.nhsdirect.nhs.uk Please use the space below to write down any questions you may want to ask Wet Age-Related Macular Degeneration - Information For Patients Version 1 March 2009 Next Review Date Nov 2010 Page 6 of 6