Supplementary Table 1
advertisement
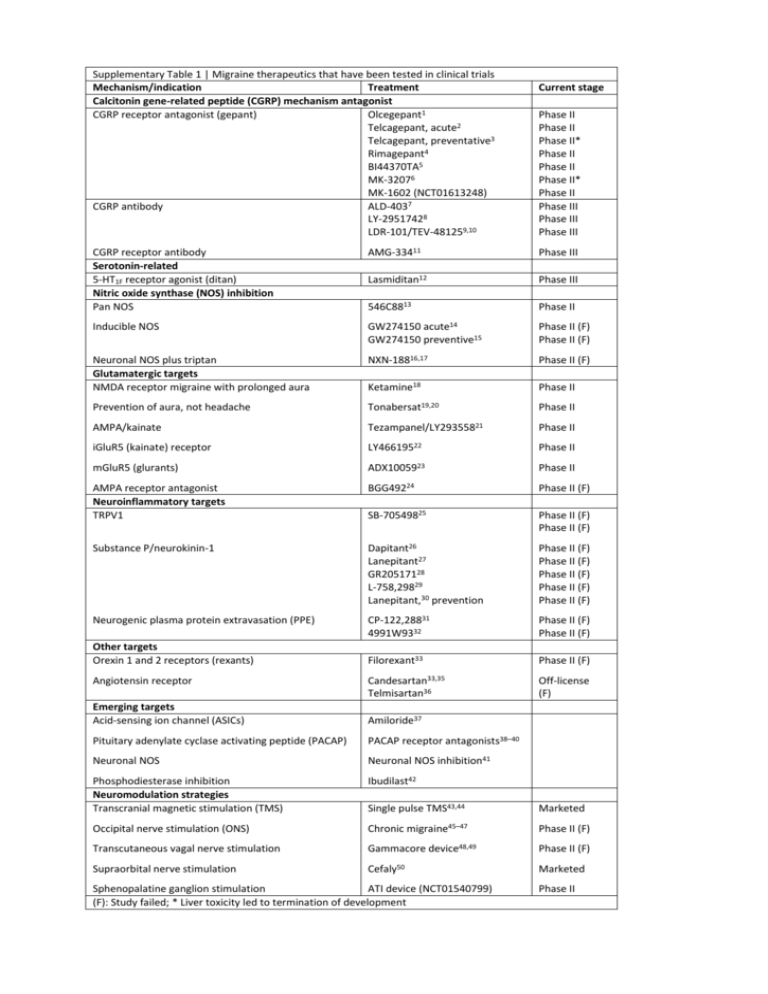
Supplementary Table 1 | Migraine therapeutics that have been tested in clinical trials Mechanism/indication Treatment Calcitonin gene-related peptide (CGRP) mechanism antagonist CGRP receptor antagonist (gepant) Olcegepant1 Telcagepant, acute2 Telcagepant, preventative3 Rimagepant4 BI44370TA5 MK-32076 MK-1602 (NCT01613248) CGRP antibody ALD-4037 LY-29517428 LDR-101/TEV-481259,10 Current stage Phase II Phase II Phase II* Phase II Phase II Phase II* Phase II Phase III Phase III Phase III CGRP receptor antibody Serotonin-related 5-HT1F receptor agonist (ditan) Nitric oxide synthase (NOS) inhibition Pan NOS AMG-33411 Phase III Lasmiditan12 Phase III 546C8813 Phase II Inducible NOS GW274150 acute14 GW274150 preventive15 Phase II (F) Phase II (F) Neuronal NOS plus triptan Glutamatergic targets NMDA receptor migraine with prolonged aura NXN-18816,17 Phase II (F) Ketamine18 Phase II Prevention of aura, not headache Tonabersat19,20 Phase II AMPA/kainate Tezampanel/LY29355821 Phase II iGluR5 (kainate) receptor LY46619522 Phase II mGluR5 (glurants) ADX1005923 Phase II AMPA receptor antagonist Neuroinflammatory targets TRPV1 BGG49224 Phase II (F) SB-70549825 Phase II (F) Phase II (F) Substance P/neurokinin-1 Dapitant26 Lanepitant27 GR20517128 L-758,29829 Lanepitant,30 prevention Phase II (F) Phase II (F) Phase II (F) Phase II (F) Phase II (F) Neurogenic plasma protein extravasation (PPE) CP-122,28831 4991W9332 Phase II (F) Phase II (F) Filorexant33 Phase II (F) Candesartan33,35 Telmisartan36 Off-license (F) Other targets Orexin 1 and 2 receptors (rexants) Angiotensin receptor Emerging targets Acid-sensing ion channel (ASICs) Amiloride37 Pituitary adenylate cyclase activating peptide (PACAP) PACAP receptor antagonists38–40 Neuronal NOS Neuronal NOS inhibition41 Phosphodiesterase inhibition Neuromodulation strategies Transcranial magnetic stimulation (TMS) Ibudilast42 Single pulse TMS43,44 Marketed Occipital nerve stimulation (ONS) Chronic migraine45–47 Phase II (F) Transcutaneous vagal nerve stimulation Gammacore Supraorbital nerve stimulation Cefaly50 device48,49 Sphenopalatine ganglion stimulation ATI device (NCT01540799) (F): Study failed; * Liver toxicity led to termination of development Phase II (F) Marketed Phase II 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. Olesen, J. et al. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. New England Journal of Medicine 350, 1104–1110 (2004). Ho, T.W. et al. Efficacy and tolerability of MK-0974 (telcagepant), a new oral antagonist of calcitonin gene-related peptide receptor, compared with zolmitriptan for acute migraine: a randomised, placebo-controlled, paralleltreatment trial. Lancet 372, 2115–2123 (2008). Ho, T.W. et al. Randomized controlled trial of the CGRP receptor antagonist telcagepant for migraine prevention. Neurology (Minneap.) 83, 958–966 (2014). Marcus, R. et al. BMS-927711 for the Acute Treatment of Migraine: A Double-Blind, Randomized, PlaceboControlled, Dose-Ranging Trial. Cephalalgia 33, 94 (2013). Diener, H.-C. et al. BI 44370 TA, an oral CGRP antagonist for the acute treatment of migraine attacks: results from a phase II study. Cephalalgia 31, 573–584 (2011). Hewitt, D.J. et al. Randomized controlled trial of the CGRP receptor antagonist, MK-3207, in the acute treatment of migraine. Cephalalgia 31, 712–722 (2011). Dodick, D.W. et al. Randomized, Double-blind, Placebo-controlled, Phase II Trial of ALD403, an anti-CGRP peptide antibody in the prevention of frequent episodic migraine. Lancet Neurology 13, 1100–1107 (2014). Dodick, D.W. et al. CGRP Monoclonal Antibody LY2951742 for the Prevention of Migraine: A Phase 2, Randomized, Double-Blind, Placebo-Controlled Study. Lancet Neurology 13, 885–892 (2014). Bigal, M.E. et al. Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of high-frequency episodic migraine: a multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurology, in press (2015). Bigal, M.E. et al. Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of chronic migraine: a multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurology, in press (2015). Lenz, R. et al. Results of a randomized, double-blind, placebo controlled, phase 2 study to evaluate the efficacy and safety of AMG 334 for the prevention of episodic migraine. Cephalalgia 35, 5 (2015). Farkkila, M. et al. Efficacy and tolerability of lasmiditan, an oral 5-HT(1F) receptor agonist, for the acute treatment of migraine: a phase 2 randomised, placebo-controlled, parallel-group, dose-ranging study. Lancet Neurol 11, 405–413 (2012). Lassen, L.H., Ashina, M., Christiansen, I., Ulrich, V. & Olesen, J. Nitric oxide synthesis inhibition in migraine. The Lancet 349, 401–402 (1997). Palmer, J.E. et al. A randomised, single-blind, placebo-controlled, adaptive clinical trial of GW274150, a selective iNOS inhibitor, in the treatment of acute migraine. Cephalalgia 29, 124 (2009). Hoye, K. et al. Efficacy and tolerability of the iNOS inhibitor GW274150 administered up to 120 mg daily for 12 weeks in the prophylactic treatment of migraine. Cephalalgia 29, 132 (2009). Medve, R.A. & Andrews, J.S. Effects of fixed dose combination of nNOS inhibition and 5HT agonism on progression of migraine with and without aura. Cephalalgia 29, 126 (2009). Hougaard, A., Hauge, A.W., Guo, S. & Tfelft-Hansen, P. The nitric oxide synthase inhibitor and serotonin-receptor agonist NXN-188 during the aura phase of migraine with aura: a randomized, double-blind, placebo-controlled cross-over study. Scandinavian Journal of Pain 4, 48–52 (2013). Afridi, S., Giffin, N.J., Kaube, H. & Goadsby, P.J. A randomized controlled trial of intranasal ketamine in migraine with prolonged aura. Neurology 80, 642–647 (2013). Hauge, A.W., Asghar, M.S., Schytz, H.W., Christensen, K. & Olesen, J. Effects of tonabersat on migraine with aura: a randomised, double-blind, placebo-controlled crossover study. Lancet Neurology 8, 718–723 (2009). Goadsby, P.J., Ferrari, M.D., Csanyi, A., Olesen, J. & Mills, J.G. Randomized double blind, placebo-controlled proof-of-concept study of the cortical spreading depression inhibiting agent tonabersat in migraine prophylaxis. Cephalalgia 29, 742–750 (2009). Sang, C.N. et al. LY293558, a novel AMPA/GluR5 antagonist, is efficacious and well-tolerated in acute migraine. Cephalalgia 24, 596–602 (2004). Johnson, K.W. et al. in Innovative Drug Development for Headache Disorders (eds. Olesen, J. & Ramadan, N.) 185–194 (Oxford University Press, Oxford, 2008). Varon, S. F. et al. Healthcare resource utilization patterns among individuals with chronic migraine (CM) and episodic migraine (EM). Cephalalgia 29, 60–61 (2009). Gomez-Mancilla, B. et al. Randomized, multicenter trial to assess the efficacy, safety and tolerability of a single dose of a novel AMPA receptor antagonist BGG492 for the treatment of acute migraine attacks. Cephalalgia 34, 103–113 (2014). Chizh, B. et al. A randomised, two-period cross-over study to investigate the efficacy of the Trpv1 antagonist SB705498 in acute migraine. Eur J Pain 13, S202a–S202 (2009). Diener, H.-C. & The RPR100893 Study Group. RPR100893, a substance-P antagonist, is not effective in the treatment of migraine attacks. Cephalalgia 23, 183–185 (2003). Goldstein, D.J. et al. Ineffectiveness of neurokinin-1 antagonist in acute migraine: a crossover study. Cephalalgia 17, 785–790 (1997). Connor, H.E. et al. Clinical evaluation of a novel, potent, CNS penetrating NK1 receptor antagonist in the acute treatment of migraine. Cephalalgia 18, 392 (1998). Norman, B., Panebianco, D. & Block, G.A. A placebo-controlled, in-clinic study to explore the preliminary safety and efficacy of intravenous L-758,298 ( a prodrug of the NK1 receptor antagonist L-754,030) in the acute treatment of migraine. Cephalalgia 18, 407 (1998). Goldstein, D.J. et al. Lanepitant, an NK-1 antagonist, in migraine prevention. Cephalalgia 21, 102–106 (2001). 31. 32. 33. 34. 35. 36. 37. 38. 39. 40. 41. 42. 43. 44. 45. 46. 47. 48. 49. 50. Roon, K.I. et al. No acute antimigraine efficacy of CP-122,288, a highly potent inhibitor of neurogenic inflammation: results of two randomized double-blind placebo-controlled clinical trials. Annals of Neurology 47, 238–241 (2000). Earl, N.L., McDonald, S.A., Lowy, M.T. & 4991W93 Investigator Group. Efficacy and tolerability of the neurogenic inflammation inhibitor, 4991W93, in the acute treatment of migraine. Cephalalgia 19, 357 (1999). Chabi, A. et al. Randomized controlled trial of the orexin receptor antagonist filorexant for migraine prophylaxis. Cephalalgia 35, 379–388 (2015). Stovner, L.J. et al. A comparative study of candesartan versus propranolol for migraine prophylaxis: A randomised, triple-blind, placebo-controlled, double cross-over study. Cephalalgia 34, 523–532 (2014). Tronvik, E., Stovner, L.J., Helde, G., Sand, T. & Bovim, G. Prophylactic treatment of migraine with an angiotensin II receptor blocker: a randomized controlled trial. Journal of the American Medical Association 289, 65–69 (2003). Diener, H.C. et al. Telmisartan in migraine prophylaxis: a randomized, placebo-controlled trial. Cephalalgia 29, 921–927 (2009). Holland, P.R. et al. Acid-sensing ion channel-1: a novel therapeutic target for migraine with aura. Annals of Neurology 72, 559–563 (2012). Amin, F.M. et al. Investigation of the pathophysiological mechanisms of migraine attacks induced by pituitary adenylate cyclase-activating polypeptide-38. Brain 137, 779–794 (2014). Zagami, A.S., Edvinsson, L. & Goadsby, P.J. Pituitary adenylate cyclase activating polypeptide and Migraine. Annals of Clinical and Translational Neurology 1, 1036–1040 (2014). Akerman, S. & Goadsby, P.J. Neuronal PAC1 receptors mediate delayed activation and sensitization of trigeminocervical neurons: relevance to migraine. Science Translational Medicine 7, 308ra157 (2015). Akerman, S., Williamson, D.J., Kaube, H. & Goadsby, P.J. Nitric oxide synthase inhibitors can antagonise neurogenic and calcitonin gene-related peptide induced dilation of dural meningeal vessels. British Journal of Pharmacology 137, 62–68 (2002). Laursen, J.C. et al. Nitric oxide release from trigeminal satellite glial cells is attenuated by glial modulators and glutamate. Int J Physiol Pathophysiol Pharmacol 5, 228–238 (2013). Lipton, R.B. et al. Single-pulse transcranial magnetic stimulation for acute treatment of migraine with aura: a randomised, double-blind, parallel-group, sham-controlled trial. Lancet Neurol 9, 373–380 (2010). Bhola, R. et al. Single-pulse transcranial magnetic stimulation (sTMS) for the acute treatment of migraine: Evaluation of outcome data for the UK post market pilot program. Journal of Headache and Pain 16, 51 (2015). Saper, J. et al. Occipital nerve stimulation for the treatment of intractable chronic migraine headache: ONSTIM feasibility study. Cephalalgia 41, 271–285 (2011). Silberstein, S. et al. The safety and efficacy of occipital nerve stimulation for the management of chronic migraine. Cephalalgia 32, 1165–1179 (2012). Lipton, R.B. et al. PRISM study: occipital nerve stimulation for treatment-refractory migraine. Cephalalgia 29, 30 (2009). Goadsby, P.J., Grosberg, B.M., Mauskop, A. & Cady, R. Effect of non-invasive vagus nerve stimulation on acute migraine: an open label pilot study. Cephalalgia 34, 986–993 (2014). Silberstein, S.D. et al. Non-invasive Vagus Nerve Stimulation for Chronic Migraine Prevention in a Prospective, Randomized, Sham-Controlled Pilot Study (the EVENT Study): Report from the Double-blind Phase. Headache 54, 1426 (2014). Schoenen, J. et al. Migraine prevention with a supraorbital transcutaneous stimulator: a randomized controlled trial. Neurology 80, 697–704 (2013).