Tentative Protocols for the Upcoming Year
advertisement
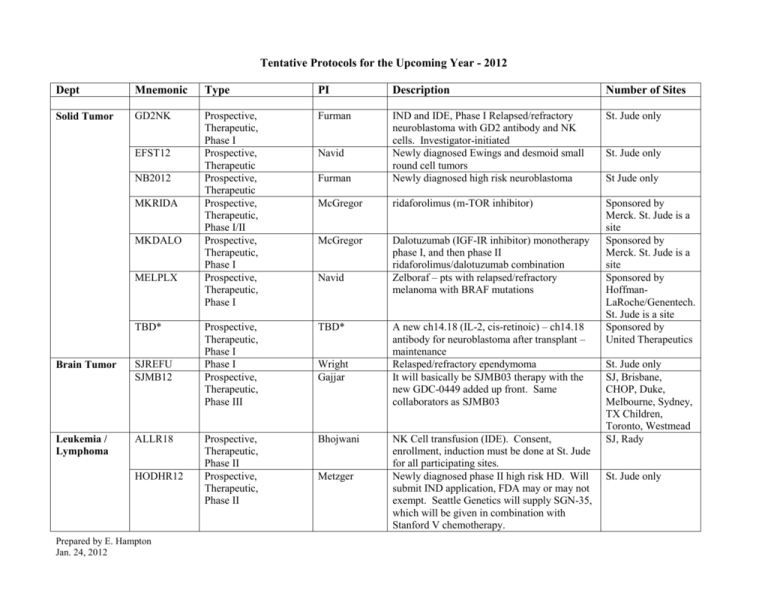
Tentative Protocols for the Upcoming Year - 2012 Dept Mnemonic Type PI Description Number of Sites Solid Tumor GD2NK Prospective, Therapeutic, Phase I Prospective, Therapeutic Prospective, Therapeutic Prospective, Therapeutic, Phase I/II Prospective, Therapeutic, Phase I Prospective, Therapeutic, Phase I Furman St. Jude only Furman IND and IDE, Phase I Relapsed/refractory neuroblastoma with GD2 antibody and NK cells. Investigator-initiated Newly diagnosed Ewings and desmoid small round cell tumors Newly diagnosed high risk neuroblastoma McGregor ridaforolimus (m-TOR inhibitor) McGregor Dalotuzumab (IGF-IR inhibitor) monotherapy phase I, and then phase II ridaforolimus/dalotuzumab combination Zelboraf – pts with relapsed/refractory melanoma with BRAF mutations Prospective, Therapeutic, Phase I Phase I Prospective, Therapeutic, Phase III TBD* Sponsored by Merck. St. Jude is a site Sponsored by Merck. St. Jude is a site Sponsored by HoffmanLaRoche/Genentech. St. Jude is a site Sponsored by United Therapeutics Prospective, Therapeutic, Phase II Prospective, Therapeutic, Phase II Bhojwani EFST12 NB2012 MKRIDA MKDALO MELPLX TBD* Brain Tumor SJREFU SJMB12 Leukemia / Lymphoma ALLR18 HODHR12 Prepared by E. Hampton Jan. 24, 2012 Navid Navid Wright Gajjar Metzger A new ch14.18 (IL-2, cis-retinoic) – ch14.18 antibody for neuroblastoma after transplant – maintenance Relasped/refractory ependymoma It will basically be SJMB03 therapy with the new GDC-0449 added up front. Same collaborators as SJMB03 NK Cell transfusion (IDE). Consent, enrollment, induction must be done at St. Jude for all participating sites. Newly diagnosed phase II high risk HD. Will submit IND application, FDA may or may not exempt. Seattle Genetics will supply SGN-35, which will be given in combination with Stanford V chemotherapy. St. Jude only St Jude only St. Jude only SJ, Brisbane, CHOP, Duke, Melbourne, Sydney, TX Children, Toronto, Westmead SJ, Rady St. Jude only Dept Mnemonic Type PI Description Number of Sites Leukemia / Lymphoma RET6 Prospective, Therapeutic Brennan Newly diagnosed intra-ocular retinoblastoma. Primary objective is biologic, but will enroll all patient all stages with intraocular retinoblastoma presenting to St. Jude. St. Jude only ALL1121 Prospective, Therapeutic Bhojwani BiTE study COG, but industry sponsored and monitored RESPV2 Prospective, Non-Therapeutic Srinivasan A prospective 2 year study for viral load determination of adenovirus, rhinovirus, RSV, parainfluenza, influenza and CMV in NP wash samples from allogeneic HSCT patients with symptomatic URTI and/or LRTI. We propose to start this off at St Jude expecting to accrue 100 patients over 2 years (based on RESPVI numbers), and hopefully include 2 other BMT sites including adult centers, for a total expected accrual of about 300-400 patients and 1000 samples. UCBT with ex vivo expanded NK cells for hematologic malignancies. Feasibility pilot study to examine the acceptability of and initial response to growth hormone replacement therapy for survivors of childhood leukemia who were treated with cranial radiation and who have adult growth hormone deficiency and poor quality of life. In this pilot study, participants will be randomized to receive either growth hormone replacement or placebo for the 15 month trial. 2 Pharma/Industry 1 PHACS network 2 SJ Investigator 3 ATN network 3 IMPAACT network St. Jude and 2 sites TGT UCBNK Epidemiology GROWTH Infectious Diseases Prepared by E. Hampton Jan. 24, 2012 Dallas Pilot Chemaitilly St. Jude only St. Jude only St. Jude is a site St. Jude is a site St. Jude only St. Jude is a site St. Jude is a site Dept Mnemonic Type PI Description Number of Sites Psychology MEMFX3 Prospective Conklin Genetic Polymorphisms as predictors of response to MPH on MEMFX2 DC-TRN (grant: ACCL10P1) CSqHPV Prospective Conklin Prophylactic CogMed training during RT Likely SJ only, but MEMFX2 also included Duke and Med. Univ. S. Carolina (possibly) SJ is one of 8 participating sites Prospective, therapeutic Klosky ACYSCD Prospective, nontherapeutic Porter Enuresis and Pica in Sickle Cell Disease Prospective, nontherapeutic Porter BMTPE3 Prospective, nontherapeutic Phipps SMTEXT Prospective, nontherapeutic Tyc Quadrivalent Human Papillomavirus (qHPV) Vaccine in Cancer Survivors: Cross Sectional Survey and Phase II Open-Label Vaccine Trial This study entails 6 focus groups of adolescents with Sickle Cell Disease, their caregivers, and young adults ages 18-25 who have transitioned to adult providers. We will be identifying their perceptions about transition, transition readiness, current disease management skills, and perceptions of the components of the current transition program. Patients with SCD ages 6-18 and caregivers will complete questionnaires about enuresis and pica behaviors and interventions utilized, in addition to measures of family stress, treatment acceptability, emotional functioning. Medical information from MILLI will be gathered. Cognitive, Academic And Psychosocial Functioning In Long-Term Survivors Of Pediatric Stem Cell Transplantation The Feasibility Of Text Messaging To Assess Secondhand Smoke Exposure Among Youngsters With Cancer And Sickle Cell Disease Psychology Prepared by E. Hampton Jan. 24, 2012 This is a Consortium for Pediatric Interventional Research study SJ only SJ only SJ only SJ only Dept Psychology Mnemonic SMOBAN Type Prospective, nontherapeutic PI Tyc Hematology TBD* Prospective, therapeutic Nienhuis SRVIRON Prospective, nontherapeutic Nottage TBD* Prospective, nontherapeutic Nottage TBD* - To be determined Prepared by E. Hampton Jan. 24, 2012 Description Number of Sites An assessment of preferences for smoking ban SJ only vs. smoking cessation interventions among families of children with cancer and sickle cell disease An open label dose-escalation study of a self- SJ Only complementary adeno-associated viral vector for gene transfer in subjects with Galactosialidosis” during the next 12 -18 months. A cross-sectional, non-therapeutic study of iron overload in long-term cancer survivors. This will be in conjunction with the Epi department and will involve clinical evaluation, blood tests, Echos, and MRI in cancer survivors. Longitudinal cohort in sickle cell disease: non-therapeutic cohort study of patients at SJCRH (pediatric) and Methodist (adult patients) with sickle cell disease. The aim will be to establish a long-term cohort and collect clinical data on which to base further research. Similar to SJLIFE. SJ Only SJ and Methodist Dept Hematology Mnemonic TBD* Type Prospective, nontherapeutic PI Nottage Description Translational Study of the Efficacy of Novel Compounds in Erythrocytes Affected by Pyruvate Kinase Deficiency or Chronic Hemolytic Processes: The primary aim of this study is to explore the activity of several novel compounds currently in development at Agios Pharmaceuticals in purified erythrocytes from patients with PKDassociated or other chronic hemolytic anemia’s. Non-therapeutic, one time blood draws. Number of Sites SJ only SICHA Prospective, nontherapeutic Nottage Splenectomy in Congenital Hemolytic Anemia): The aim of the study is to record in a internet-accessible disease registry relevant clinical outcomes of children with congenital hemolytic anemias who are undergoing total or partial splenectomy. Outcomes will include clinical events, hematologic results, perioperative outcomes, imaging data, and adverse events. No specific surgical procedure, medical tests, or therapies will be mandated by this study. SJ only EPIC Prospective, therapeutic Estepp Intravenous Enoxaparin in Pediatric Thromboembolism: A Dose Finding and Pharmacodynamics Study (EPIC). Determine a dosage (mg/kg/dose) of enoxaparin administered intravenously that provides therapeutic peak anti-Xa levels without excessive clinical toxicity. SJ only HUG3 Prospective, therapeutic Wing Protocol design similar to HUG2 SJ only Prepared by E. Hampton Jan. 24, 2012





