Materials and Methods. (doc 40K)
advertisement

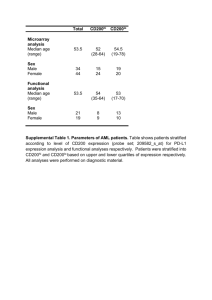
SUPPLEMENTAL METHODS. Supplemental methods 1. PET analyses of HSV1-TK expression The animals were fasted overnight before the PET study. On the day of the PET imaging, anesthesia was initially induced with ketamine (0.1 mg/kg im, imalgene 1000, Merial, France) and imidazolam (1.84 mg/kg im, Dormicum, Roche Farma, Spain) to allow the transport of the animals to the PET Laboratory. Anesthesia during the scan was maintained with ketamine (0.05 mg/kb, im) and imidazolam (0.92 mg/kg im). Scans were performed in a small animal dedicated Mosaic PET scanner (Philips) with an11.9 cm axial and 12.8 cm transaxial field of view (FOV). Dynamic and transmission studies have been described[13]. 18F-FHBG was synthesized as reported and specific accumulation of this radioactive tracer in the liver of treated macaques was measured as previously reported[13]. Supplemental methods 2. FACS analysis Total blood (100 L) was stained with anti-monkey CD3-APC (clone 1OD12, Miltenyi), anti-human CD8-PeCy5 (clone OKT-8, BD Biosciences), anti-human CD19PE (clone J4.119, Beckman Coulter) and anti-human CD20-FITC (clone 2H7, BD Biosciences) mAbs for 45 min a 4ºC. Monoclonal antibodies OKT-8, J4.119, and 2H7 had been reported to cross-react with cynomolgus lymphocytes. Human Beriglobine (10 g/mL) was added during stainings to avoid unspecific binding. At the end of the labeling, red cells were lysed without previous washing by adding 1 mL of 1 BD FACS Lysing solution (BD Biosciences) to each sample. After 5 min of incubation at room temperature, cells were pelleted and washed once before acquisition on a FACSCalibur cytometer (BD Biosciences). FACS data were analyzed using FlowJo 2 program (Tree Star, Inc). The percentages of B (CD19+CD20+), CD4 (CD3+CD8-) and CD8 (CD3+CD8+) lymphocytes were referred to the total leukocyte population gated by Forward-scatter (size) and Side-scatter (morphology). To estimate the cell number/mL of peripheral blood of each lymphocyte population, we applied the following formula: (% of a particular lymphocyte population) (number of total leucocytes per mL of blood) / 100. The number of total leukocytes per mL of blood was determined by checking the total number of isolated leukocytes (purified and counted as detailed below) to the starting peripheral blood volume. Supplemental methods 3. Measurement of cellular immune responses against adenoviral vector. Monkey leukocytes were purified from peripheral blood by centrifugation through Ficoll-Hypaque (GE Healthcare) (20 min at 600 g and 20ºC). Contaminating erythrocytes were lysed by treatment with ACK buffer (0.15M NH4CL, 10 mM KHCO3, 0.1mM Na2EDTA). The total number of isolated leukocytes was determined by counting the cells in a Z2 Coulter Counter (Beckman Coulter). In order to measure proliferation by methyl-3HThymidine incorporation, isolated leukocytes were cultured at a concentration of 5 x 105 cells/mL with culture medium alone X-vivo medium (BioWhittaker) supplemented with 2 mM glutamax (Invitrogen), and 1% penicillin/streptomycin (Invitrogen) or culture medium containing serial dilutions of adenovirus capsid protein from 10-0.01 g/mL. Six hours before the 72h of culture, cells were pulsed with 1 Ci/well of tritiated thymidine and cultured again. At the end of this labeling time cells were harvested and methyl-3HThymidine incorporation was determined in a scintillantion counter (Topcount). Stimulation Index (S.I.) was defined as the mean counts per minute (cpm) of the response of the antigenstimulated cells divided by the mean cpm of the response of cells cultured without 3 antigen. A mix of the phorbol ester 12-myristate- 13-acetate (PMA) (0.01 g/mL) and ionomicine (1 g/mL) was used as a positive control to assess the ability of cells to respond to mitogens. Viral capsids had been harvested from the upper band adenovirus purifications after centrifugation at 25,000 rpms for 12h in a CsCl gradient corresponding to 1.25 g/ml density. To determine specific proliferation of CD8 and CD4 T cells, isolated leukocytes were resuspended in PBS at a final concentration of 5-10 x 106 cells/mL and labeled with 5(and -6)-carboxyfluorescein diacetate succinimidyl ester (CFSE) (SIGMA) (1.25 M) for 15 minutes at RT. CFSE-labelled cells were washed and subsequently resuspended in fetal calf serum-supplemented medium RPMI-glutamax medium (Invitrogen) supplemented with 10% FCS (Sigma) and 1% penicillin/streptomycin (Invitrogen) at a concentration of 1 x 106 cells/mL in the absence or presence of adenovirus capsid protein (5 g/mL). After 6 days of culture, cells were harvested, stained with antimonkey CD3-APC and anti-human CD8-PECy5 mAb in the presence of Beriglobine and finally acquired on a FACSCalibur. Proliferation of CD8 and CD4 T cells was assessed by CFSE dilution gating on CD3+CD8+ cells (CD8 T cells) and on CD3+CD8(CD4 T cells) respectively. Supplemental methods 4. Western blot analysis of liver biopsies. Twenty or fifty μg of protein were electrophoresed on a 12% SDS-PAGE and transferred onto nitrocellulose membranes using an electroblot system (Biorad). The membrane was incubated with a 1:300 dilution of the polyclonal rabbit anti-TK sera (provided by Dr WC Summers, Yale University) and 1:400 dilution of anti-rabbit IgG horseradish-peroxidase conjugate (Goat anti-rabbit, GH/P, Ref. 170-6515, Biorad). Light emission after the addition of the western lightning chemiluminescence reagent 4 (NLE 101, Perkin Elmer) was measured using an ImageQuant RT ECL (Amersham Biosciences). Supplemental methods 5. Immunohistochemistry of liver biopsies. Serial paraffin sections were prepared and antigen retrieval was performed for 20 min at 95 ºC in 0.01 M Tris-1 mM EDTA buffer (pH=9) in a Pascal pressure chamber (S2800, Dako). Samples were stained using a polyclonal rabbit anti-TK serum (1:8000; provided by Dr WC Summers, Yale University) or a monoclonal mouse anti-human CD68 (clone KP1, Code No. F7135, Dako, 1:400). The secondary labelled antibodies used were goat anti-rabbit (K4003, Dako) or goat anti-mouse (K1001, Dako). Peroxidase was developed using DAB+ (K3468, Dako). Sections were lightly counterstained with hematoxylin and mounted. Quantitation of TK+ cells by immunohistochemistry out of 5,000 counted cells per hepatic lobe from the indicated macaques. Morphology distinguished hepatocytes from Kupffer cells and the distinction of TK+ cells was taken into account. Supplemental methods 6. Construction of SFV-hisTK vector An expression system based on a Semliki forerest virus vector was used. Plasmid pSFVb1-2A has been previously described and was kindly provided by Dr. P. Liljeström (Karolinska Institute, Stockholm) [27]. For the generation of pSFV-hisTK a PCR fragment of 1.1 Kb containing the sequence of HSV-tk gene was amplified from plasmid pSFV-enhTK, which had previously been generated by subcloning HSV-tk gene from pMV60/tk (Peñuelas et al., 2005) into pSFV-b12A. Briefly, HSV-tk was amplified from pSFV-TK by using primers 5´- TAATACCCGGGCACCACCACCACCACCACGCTTCGTACCCCGGCCATCAACA 5 C-3´ and 5´-TCCACCCGGGTCAGTTAGCCTCCCCCATCTCTCGGGCAAACG-3´ which hybridize with the 5´ and 3´ ends of the HSV-tk gene, respectively (in italics), contain Xma I sites (in bold) and a sequence coding for 6 histidines (underlined) preceding the beginning of HSV-tk sequence. This PCR fragment was digested with Xma I and inserted into Xma I site in pSFVb1-2A, generating pSFV-hisTK. Transfection of cells for TK production Plasmid pSFV-hisTK was linearized by digestion with SpeI and transcribed in the presence of cap analog (New England Biolabs, USA) by using SP6 polymerase (Amersham-Pharmacia, USA-Sweden) as described [28]. In vitro synthesized RNAs was transfected into BHK-21 cells by electroporation as described previously (Liljestrom and Garoff, 1994). For TK production a total of 108 BHK-21 cells were electroporated with SFV-hisTK RNA (25 μg of RNA for each 107 cells), and incubated for 24h at 33º. Cells were washed twice with PBS and lysed in a total volume of 1 ml of lysis buffer containing 50mM Tris-ClH pH 8, 500mM ClNa and Complete Protease Inhibitor Cocktail (Roche, Germany). Purification from the lysates was performed in a Fast Protein Liquid Chromatography with HitrapAffinity Columns (His-Trap FFC rude, GEHealthcare) and imidazole gradient.