Rodent Blood Collection Procedures
advertisement
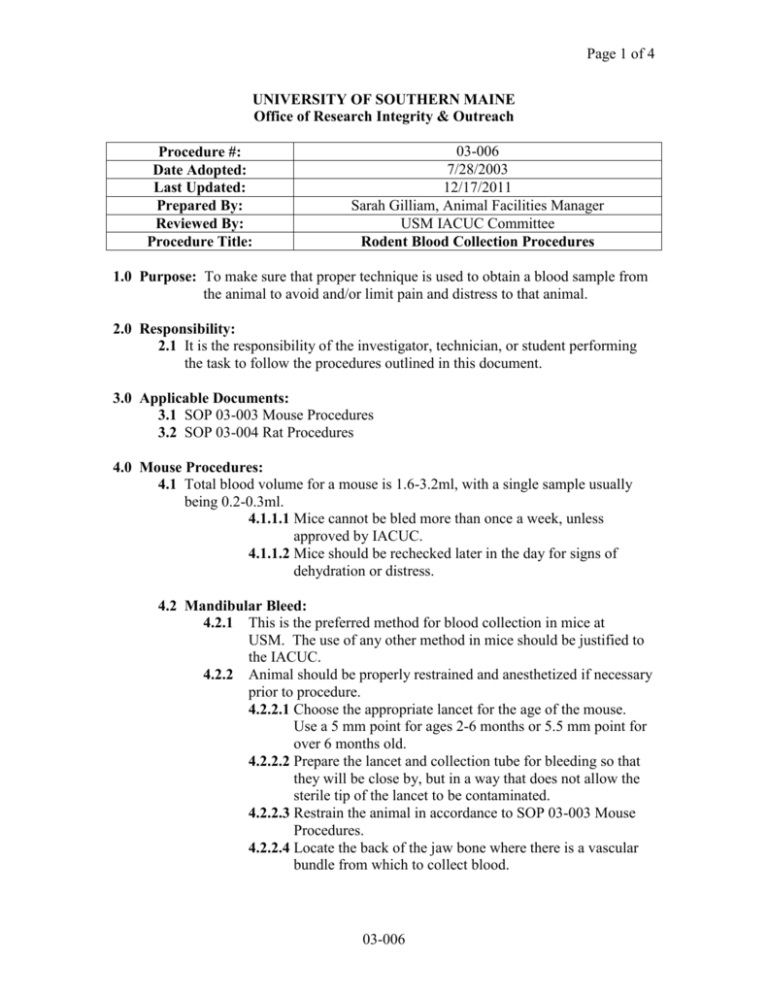
Page 1 of 4 UNIVERSITY OF SOUTHERN MAINE Office of Research Integrity & Outreach Procedure #: Date Adopted: Last Updated: Prepared By: Reviewed By: Procedure Title: 03-006 7/28/2003 12/17/2011 Sarah Gilliam, Animal Facilities Manager USM IACUC Committee Rodent Blood Collection Procedures 1.0 Purpose: To make sure that proper technique is used to obtain a blood sample from the animal to avoid and/or limit pain and distress to that animal. 2.0 Responsibility: 2.1 It is the responsibility of the investigator, technician, or student performing the task to follow the procedures outlined in this document. 3.0 Applicable Documents: 3.1 SOP 03-003 Mouse Procedures 3.2 SOP 03-004 Rat Procedures 4.0 Mouse Procedures: 4.1 Total blood volume for a mouse is 1.6-3.2ml, with a single sample usually being 0.2-0.3ml. 4.1.1.1 Mice cannot be bled more than once a week, unless approved by IACUC. 4.1.1.2 Mice should be rechecked later in the day for signs of dehydration or distress. 4.2 Mandibular Bleed: 4.2.1 This is the preferred method for blood collection in mice at USM. The use of any other method in mice should be justified to the IACUC. 4.2.2 Animal should be properly restrained and anesthetized if necessary prior to procedure. 4.2.2.1 Choose the appropriate lancet for the age of the mouse. Use a 5 mm point for ages 2-6 months or 5.5 mm point for over 6 months old. 4.2.2.2 Prepare the lancet and collection tube for bleeding so that they will be close by, but in a way that does not allow the sterile tip of the lancet to be contaminated. 4.2.2.3 Restrain the animal in accordance to SOP 03-003 Mouse Procedures. 4.2.2.4 Locate the back of the jaw bone where there is a vascular bundle from which to collect blood. 03-006 Page 2 of 4 4.2.2.5 Once the correct location is found, use the lancet to quickly puncture the area (no deeper than the lancet point) and withdraw the lancet. Dispose of the lancet in a sharps waste container. 4.2.2.6 As the blood appears from the puncture, use the collection tube to harvest it, being careful not to exceed the recommended 10-15% of total blood volume or 1% of body weight. 4.2.2.7 Once the sample is collected, use gauze to press against the site in order to stop the bleeding. 4.2.2.8 Place the mouse back in its cage and observe until fully recovered from the anesthesia (if used). Note on the cage card the date of the procedure. 4.3 Retro-orbital Bleed: 4.3.1 Animal should be properly restrained and anesthetized. 4.3.1.1 Make sure to clean the anesthesia chamber before & after each use as well as between animals to prevent the spread of pathogens. 4.3.1.2 Insert the end of a micro-hematocrit tube into the retroorbital sinus via the medial canthus. 4.3.1.3 After the tube is full, either place into a sample tube or insert the tube into sealing compound. 4.3.1.4 Label the sealing compound or sample tube with the animal information and date. 4.3.1.5 Do not collect more than 3 tubes per mouse, unless previously approved by IACUC. 4.3.1.6 After collection, gently wipe area with clean gauze & observe for additional bleeding. 4.3.1.6.1 If bleeding continues use clean gauze to apply gentle pressure to the area until the bleeding stops. 4.3.1.7 Note on the cage card the date and the procedure done. 4.4 Tail Bleed: 4.4.1 A 26-28 gauge needle should be used. 4.4.2 Animal should be properly anesthetized or restrained (in a rodent restrainer). 4.4.3 Either the ventral caudal artery or the lateral caudal veins may be used for collecting very small amounts of blood. 4.4.4 Warming the animal’s tail by placing it into a cup of warm water can be done to improve circulation within the tail. 4.4.4.1 Be sure to test the temperature of the water, if it is too hot for the technician then it is too hot for the animal. 4.4.5 Disinfect the site with an appropriate disinfectant. 03-006 Page 3 of 4 4.4.6 4.4.7 Insert the needle, bevel side facing up, into the artery or vein & collect the blood from the hub of the needle with a sample tube. After removing the needle, apply gentle pressure to the vessel using clean gauze to stop the bleeding. 4.5 Cardiac Puncture: 4.5.1 This is a terminal procedure. 4.5.2 Use a 22 gauge needle. 4.5.3 Animal should be properly anesthetized or euthanized prior to procedure. 4.5.3.1 Be sure to clean the anesthesia chamber before & after each use as well as between animals to prevent the spread of pathogens. 4.5.4 Two methods: 4.5.4.1 First method is to insert through the intercostal space (between the ribs) on the left side at the PMI (Point of Maximum Intensity) of the heart beat. 4.5.4.2 Second method is to place the animal in dorsal recumbency and insert the needle through the diaphragm below the xiphoid cartilage. 4.5.4.2.1 Then direct the needle toward the PMI. 5.0 Rat Procedures: 5.1 Total blood volume for a rat is 20-40mls, with a single sample normally being 2-3mls. 5.2 Mandibular bleed: 5.2.1 This is the preferred method for blood collection in rats at USM. The use of any other method in mice should be justified to the IACUC. 5.2.2 Animal should be properly restrained and anesthetized prior to procedure. 5.2.2.1 Choose the appropriate lancet for the age of the rat. Use a 5.5-6 mm point for ages 8-12 weeks, 6-7 mm point for 1216 weeks, and a 7-8mm point for any that are over 20 weeks of age. 5.2.2.2 Prepare the lancet and collection tube for bleeding so that they will be close by, but in a way that does not allow the sterile tip of the lancet to be contaminated. 5.2.2.3 Restrain the animal in accordance to SOP 03-004 Rat Procedures. 5.2.2.4 Locate the back of the jaw bone where there is a vascular bundle from which to collect blood. 5.2.2.5 Once the correct location is found, use the lancet to quickly puncture the area (no deeper than the lancet point) and 03-006 Page 4 of 4 withdraw the lancet. Dispose of the lancet in a sharps waste container. 5.2.2.6 As the blood appears from the puncture, use the collection tube to harvest it, being careful not to exceed the recommended 10-15% of total blood volume or 1% of body weight. 5.2.2.7 Once the sample is collected, use gauze to press against the site in order to stop the bleeding. 5.2.2.8 Place the rat back in its cage and observe until fully recovered from the anesthesia. Note on the cage card the date of the procedure. 5.3 Tail Bleed: 5.3.1 Normally bled from the lateral tail vein. 5.3.2 Use a 25g needle. 5.3.3 Follow steps 4.2.2 through 4.2.7 in Section 4.0 Mouse Procedures. 5.4 Cardiac Puncture: 5.4.1 This is a terminal procedure. 5.4.2 Follow steps 4.4.2 through 4.4.4 in Section 4.0 Mouse Procedures. 03-006