Read a more detailed version of how PopART will run
advertisement

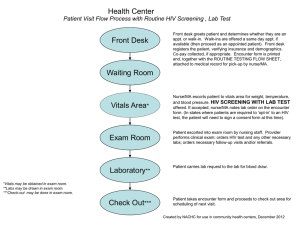
PopART: Cluster randomised trial of the impact of a combination prevention package including early antiretroviral treatment on population-level HIV incidence in Zambia and South Africa Rationale HIV prevalence and incidence remain at a very high level in many parts of Southern Africa and there is an urgent need for more effective prevention measures. Mathematical models have shown that universal voluntary HIV counselling and testing, with the offer of immediate antiretroviral treatment for those testing HIV-positive, has the potential to achieve substantial reductions in HIV incidence at population level. Testing the effectiveness of this strategy, in combination with proven preventive interventions, is a key global health priority. Study design A cluster randomised trial will be conducted in 24 clusters in Zambia and South Africa, both countries with severe generalised HIV epidemics. The cluster will be a community with a total population of at least 25,000, defined as the catchment area of specified health facilities through which services can be delivered. Of the 24 clusters, 8 will be randomly allocated to receive the PopART HIV combination prevention programme (Arm A, see below for details); 8 will receive the PopART programme but with treatment only offered to those already eligible according to national guidelines (Arm B); and the remaining 8 will continue to receive current standard of care and act as comparison communities (Arm C), to control for secular changes in HIV incidence. The 24 communities used for this trial will be the ZAMSTAR trial communities. These communities have been working with us for the past 6 years and we have good community relationships, active community advisory boards and the necessary infrastructure in place or readily developed. In addition we have unique community level estimates of HIV prevalence, HIV testing uptake, ART uptake and circumcision data for each community and this will allow us to match communities to reduce the inter-cluster variation. We also have comparable estimates of HIV incidence in a selected group of households (households of newly diagnosed TB cases) in each of these communities. A random sample of 2,500 adults will be selected from the general population of each cluster (a total of 60,000 across all 24 clusters) and followed up for 2 years to measure the impact of the intervention on HIV incidence. PopART intervention This will consist of: Intensive programme of HIV VCT, involving house-to-house visits and testing in the home or at special mobile units. Testing will be repeated at annual intervals. Offer of male circumcision (MC) to all HIV-negative men who come forward for testing (MC will also be provided to any HIV-infected men who request it). Counselling on risk reduction and condom provision will be provided as part of the VCT programme. Offer of immediate ART to all those testing HIV-positive either through the mass testing programme or through any other testing service (PICT, ANC, stand-alone VCT services etc.). We recognise that there are concerns about the feasibility of universal ART and its additional benefit over combination prevention, which is why we would include a third arm (Arm B) which would include all of the above combination package but with provision of ART according to prevailing national guidelines in Zambia and South Africa (currently defined as CD4 count < 350 cells/mm3 or WHO stage 3 or 4). In the control arm (Arm C) HIV testing will be conducted as is current standard of care in that community so will consist of a combination of PICT, ANC and stand-alone VCT approaches. Individuals who are found to be HIV positive will be referred for care according to national guidelines and HIV-negative men will be encouraged to access male circumcision again according to national guidelines. PMTCT services will be strengthened in all three study arms. Impact evaluation To measure the impact of the intervention, a population cohort consisting of a random sample of 2,500 adults aged 18-44 years will be selected from the general population of each cluster (a total of 60,000 across all clusters). A baseline survey of the cohort will be carried out at the time the intervention is initiated in the intervention clusters, to check the comparability of the intervention and control arms. Follow-up surveys of the cohort will be carried out after 1 and 2 years and used to measure the impact of the intervention on HIV incidence and other outcomes. In Arms A and B, a patient cohort, consisting of a sample of HIV-infected individuals who are detected through the testing programme, will also be followed up to measure process and other variables. Primary and secondary outcomes The primary outcome will be HIV incidence over 2 years in members of the population cohort who are HIV-negative at baseline, and will be compared in the intervention and control clusters to measure the population-level effectiveness of the PopART intervention. Secondary outcomes will also be measured in the population cohort and compared between the intervention (Arms A and B) and control (Arm C) clusters. These will include: Reported sexual risk behaviour, to test for potential risk disinhibition and to help fit mathematical models Measures of HIV-related stigma carried out as part of the qualitative research Community viral load, obtained by measuring HIV plasma viral load in cohort members who are HIV-positive at the follow-up survey Proportion of pregnant women who receive effective PMTCT services We will also measure the incidence of active TB through clinic-based surveillance; and clinical events including adverse events and other treatment outcomes Additional data will be examined on health facility workload across the different arms of the study using enhanced clinic registers. Process variables to be measured in the intervention clusters will include: Proportion accepting HIV testing and re-testing Proportional uptake of male circumcision among men testing HIV-negative Proportion of those testing HIV-positive started on ART within 3 months Proportion of those commencing ART retained on treatment and virally suppressed after 12 months Proportion of those patients not virally suppressed after 12 months who have genotypic evidence of drug resistance Sample size Preliminary mathematical modelling has shown that the full intervention can be expected to reduce HIV incidence in adults by 50-60% over two years. With 8 matched triplets of clusters and a cohort of 2,500 adults in each cluster followed up for 2 years, assuming a baseline HIV prevalence of 15%, loss to follow-up over 2 years of 20%, and a between-cluster coefficient of variation of 0.20, the trial will have 98% power of detecting a 50% impact and 88% power of detecting a 40% impact, if HIV incidence is 1.0/100py . There will also be 87% power of detecting a difference between Arms A and B, if immediate initiation of ART increases the impact from 30% to 60%. Other research components Qualitative research will be carried out to examine the acceptability of the intervention and effects on behaviour and community-level HIV-associated stigma. At the end of the testing campaign in each community, random samples of individuals who accept or decline testing will be interviewed to explore the reasons for their decisions. Similarly random samples of HIV-positive patients who are offered immediate ART and who accept or decline treatment will be interviewed to explore the reasons for their decisions. Finally, we will interview random samples of patients with good or poor adherence to ART. Data from the trial will be used to fit mathematical models that will estimate the contribution of different components of the package, and project the impact of PopART and other combination packages in a range of settings. Detailed costing studies will also be carried out in both intervention and control clusters to measure the incremental cost of the intervention. Cost data together with data from the trial will provide estimates of the cost-effectiveness of the intervention. Cost data for the different components of the intervention will also be used in combination with mathematical modelling results to estimate the cost-effectiveness of the intervention and alternative intervention packages over different time horizons in the study populations as well as in other geographical settings. Timeline The project will be carried out over a 5-year period from January 2012 to December 2016. Project team The trial will be carried out by a multi-national research consortium involving the following institutions: London School of Hygiene and Tropical Medicine: Overall coordination; epidemiological, statistical and data management support. Imperial College London: Clinical support; mathematical modelling; costing studies. ZAMBART programme, Lusaka, Zambia in partnership with Zambian Ministry of Health FHI, CIDRZ and SFH: Implementation of intervention and evaluation in Zambia clusters Desmond Tutu TB Centre, South Africa in partnership with the South African Department of Health, Provincial Department of Health: Implementation of intervention and evaluation in South African clusters Within the two study countries, the design and delivery of the intervention will be carried out in close partnership with the Ministries of Health, PEPFAR and local implementing agencies. 5 September 2011