Anesthesia, Analgesia, and Tranquilization
advertisement

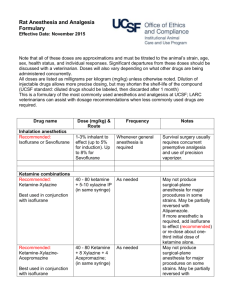
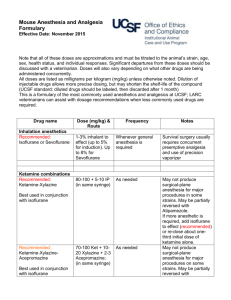
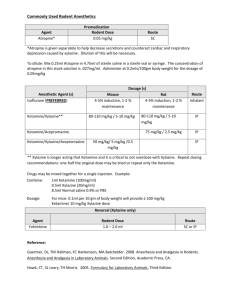
Guidelines for Anesthesia, Analgesia, and Tranquilization Policy No.: 104.01 Revision No: 4 Effective Date: (revised 02/25/14) Category: Research Guidelines I. GENERAL The Animal Welfare Act and the Public Health Service Policy on Humane Care and Use of Laboratory Animals requires that with all procedures in which the animal perceives pain, the investigator minimizes or alleviates that pain or stress or provides justification that alleviation of the pain would interfere with goals of the experimental design. II. SELECTION OF THE APPROPRIATE DRUG There are several considerations in the selection of a particular drug for an experimental procedure. The purpose of the drug (restraint, blood collection, dosing, or surgical anesthesia) will dictate the use of one type of agent over another. The type of study or procedure will require a drug which interferes the least with the experimental parameters being observed. The requirement of a post-operative analgesic may indicate the use of an anesthetic which inherently provides analgesia (such as Innovar-Vet®). The available equipment or facilities (such an inhalation equipment, scavenging devices, or hood) may dictate the use of one anesthesia over another to accommodate the facility. The use of drugs to alleviate stress and/or pain in experimental procedures may affect several parameters including the cardiovascular system, respiration, and thermoregulation in addition to the central nervous system. It is important in administration of such agents to monitor physiological parameters and maintain acceptable levels. A commonly employed device for maintaining an animal's temperature during anesthesia is a warm water heating pad. To maintain normal hydration, IV or SC fluids are administered. The experience of the individual designated to administer the drug is another important factor. Some anesthesias have a wide safety margin and can be used safely by relatively inexperienced individuals. Other agents require experienced, knowledgeable personnel to administer them due to the narrow safety margin and possible complications of their use. III. COMMONLY USED DRUGS Tranquilizers, sedatives, and hypnotics The purpose of these agents is to calm anxiety. Generally they do not provide analgesia or a loss of consciousness. Many tranquilizers are frequently used as preanesthetics in veterinary medicine with the dual purpose of calming the patient and lowering the total anesthetic required to produce anesthesia. Some examples of commonly used veterinary tranquilizers or preanesthetics are: Acetylpromazine or "Ace" is a potent neuroleptic for many animals including wild species. "Ace" has many advantages as a tranquilizer: wide safety margin, low toxicity, potent sedative action, good muscle relaxation, and can be used in combination with narcotics, dissociative anesthetics, and atropine. Ace is also a potent vasodilator, thus hypotension can be a concern. Diazepam or Valium® is a mild sedative with muscle relaxant and anticonvulsant properties. It is an excellent preanesthetic for animals with seizure disorders. Some formulations must be administered intravenously and cannot be physically mixed with atropine as precipitation results. Care must be taken to administer the drug slowly if administered IV. Xylazine or Rompun® is not a true tranquilizer due to its property of providing analgesia as well as muscle relaxation. It is used as a preanesthetic sedative. When used with a barbiturate, xylazine will reduce the total anesthesia required for the procedure. Xylazine can be used in combination with ketamine, ultrashort-acting barbiturates, halothane, or methoxyflurane. Xylazine can cause bradycardia and should be used only after premedication with atropine or glycopyrrolate. Increasing dosages increases the duration of its effects rather than the degree of sedation. Dosages vary widely between species. Analgesics Analgesic agents are used to alleviate or reduce pain. The minimizing of stress and the use of analgesia to alleviate pain is an important consideration in a research protocol. In the conduct of humane research it is important that the scientist and staff be familiar with signs exhibited by the animal which would indicate pain. Below are two tables from Lab Animal, vol. 15, #9, page 32 to assist in the evaluation of pain in rodents. The signs described may be extended to other species. Table 1. Signs for judging morbidity (disease/illness in rodents) Table 2. Signs for judging moribund condition in rodents Rapid breathing rate Breathing rate very slow, shallow, and labored Rapid weight loss Ruffled fur (rough hair coat) Hunched posture Hypothermia or hyperthermia Ulcerative dermatitis or infected tumors Inappetence Impaired ambulation (unable to reach food or water easily) Evidence of muscle atrophy or other signs of emaciation (body weight is not always proportionate) Any obvious illness including such signs as lethargy (drowsiness, aversion to activity, lack of physical or mental alertness), prolonged incontinence, bleeding, difficulty breathing, central nervous system disturbances, or chronic diarrhea or constipation Inability to remain upright Diarrhea or constipation Below is a table appearing in Lab Animal, vol. 14, #4, page 25 which describes the potential for pain in various experimental manipulations. Table 1. Post-Operative Pain Evaluation Type of Surgery Head, ear, throat, dental Ophthalmologic Orthopedic Abdominal Cardiovascular Thoracic Indications of Pain Response Rubbing, shaking, selfmutilation, depression, and reluctance to swallow, eat, drink or move Reluctance to move, scratching, rubbing Abnormal posture, abnormal gait, reluctance to move, guarding, itching, selfmutilation Guarding, abnormal posture, vomiting, anorexia Depression, reluctance to move, anxiety Changes in respiratory Expected Pain Level Duration Moderate to high Generally intermittent High Intermittent to continual Moderate Intermittent Mild to moderate Intermittent Mild to moderate Mild to Continual Continual Perirectal rate and pattern, anxiety, reluctance to move Scooting, biting, licking, self-mutilation moderate Moderate to high Intermittent to continual Some examples of commonly used analgesic agents are discussed below. Narcotics and Opiates effectively raise the pain threshold as well as altering the psychological response to pain. They provide both sedation and strong analgesia. Narcotics induce a "sleep-like" state, or stupor, through CNS depression. Disadvantages to their use are the depressant action on the CNS, cardiovascular, and respirator systems. Dosages vary considerably between species, necessitating confirmation of a suitable dose with the clinical veterinarian. Commonly used narcotics in veterinary applications are: Buprenorphine is a derivative of the morphine alkaloid, thebaine. It has profound analgesic properties when administered parenterally which last 6-12 hours. It is suitable for relief of moderate to severe pain in multiple species. Buprenorphine SR is a sustained release (SR) formulation that delivers buprenorphine in a fully biodegradable liquid polymer matrix. This formulation provides consistent 72 hour release of buprenorphine. Studies have been done in dogs, cats and laboratory animals that have shown that over the 72 hour period blood levels are greater than 1 nanogram/mL for the entire 72 hour period. A study in macaques showed this plasma concentration was maintained for 5 days. Butorphonol Tartrate is an opioid agonistantagonist which is suitable for controlling moderate to severe pain in several species including cats. Its duration of action is 4-6 hours. It causes less respiratory depression than morphine. Codeine is a less effective analgesic in animals than morphine and is rarely used. It can be used to control irritating coughs in dogs. Fentanyl is a short-acting analgesic. Effects of fentanyl are similar to those of morphine, however, respiratory center depression can be more severe and apnea may result. Meperidine (Demoral®) is one fifth as potent as morphine. Meperidine may be used in dogs, nonhuman primates, and horses. It is recommended for alleviation of short-duration post-operative pain or in situations which require repeated drug administration to control pain. Rapid IV injection may cause convulsions. Morphine is used in dogs and nonhuman primates. Adverse reactions are seen in cats, horses, and cattle including increased spinal reflexes, panting, and nausea. Nonhuman primates are reported to exhibit skin irritation on the palms and soles of their feet and a loss of appetite. Depression of gastrointestinal tract mobility is a common side effect of morphine. Morphine depresses the thermoregulatory center and animals should be maintained in a temperature controlled environment when undergoing morphine treatment. Morphine causes an increase in intraocular pressure in most species and pupillary constriction in dogs and rabbits. Duration of action of morphine is 2-4 hours. Oxymorphone as an analgesic is ten times more potent than morphine with a less depressant effect on the respiratory center and less stimulation of vomiting. Oxymorphone can cause bradycardia and auditory hypersensitivity. Pentazocine is one third as potent as morphine with mild respiratory and CNS depression. Duration of action is about one hour. Generally, it is not considered a potent analgesia and is used to control chronic or mild postsurgical pain. Tramadol Hydrochloride is an oral opioid that is currently not a scheduled drug. Although its mode of action is not completely understood at least two mechanisms seem to be applicable: binding of the parent and the M1 metabolite to u-opioid receptors and weak inhibition of reuptake of norepinephrine and serotonin. Non-Narcotic Analgesics, also classified as non-steroidal antiinflammatory agents (NSAID) generally mediate the inflammatory response through antiprostaglandins or prostaglandin synthesis inhibitors. Carprofen (Rimadyl®) has antinflammatory, analgesic, and antipyretic activity similar to other NSAIDs. It is considered to be COX-2 specific. In dogs it is given twice daily. Deracoxib (Deramaxx®) has antinflammatory, analgesic, and antipyretic activity similar to other NSAIDs. It is considered to be COX-2 specific. It is newer than carprofen and can be given to dogs once daily. Flunixin meglumine (Banamine®) is a potent injectable nonsteroidal anti-inflammatory agent with potent analgesic and fever-reducing properties. It must be used cautiously for no more than 3 days consecutively. Meloxicam (Metacam®) has antinflammatory, analgesic, and antipyretic activity similar to other NSAIDs. It is considered to be COX-2 preferential, not COX-2 specific, as at higher dosages its COX-2 specificity is diminished. Saliscylates and Acetaminophens include both aspirin and Tylenol which are effective against superficial pain, especially that of musculoskeletal origin. Acetaminophen is excessively toxic to cats due to their inability to detoxify the equivalent of a human dose. General Anesthesia General anesthesia is a state of complete unconsciousness with the following components: loss of pain perception, loss of motor control, skeletal muscle relaxation, and absence of reflex response. The unconscious state cannot be interrupted by stimulation as can the narcotic induced stupor. Termination of the unconscious state of general anesthesia is accompanied by pain perception. General anesthesia can be administered by inhalation or injection methods. Common injectable anesthetics are: Barbiturates are a group of drugs with similar chemical structures. Intermediate and long duration barbiturates are used as hypnotics, sedatives, or anti-convulsants. Barbiturates act as CNS depressants and are considered poor analgesics. Shortacting or ultra-short acting are generally acceptable as anesthetic agents. Increasing barbiturate doses will progressively increase the level of CNS depression until a state of anesthesia is reached. They are potent respiratory center depressants and can have variable effects on the cardiovascular system. There is considerable variation in dose response within and between species. Barbiturates should be administered intravenously, slowly to effect. Other methods provide less predictable effects. The administration of glucose, chloramphenicol and epinephrine will all prolong recovery from barbiturate anesthesia. Dissociative Anesthetics produce an anesthetized state characterized by moderate analgesia, muscle rigidity, loss of responsiveness to external physical stimuli, and amnesia. Eyes remain open and various reflexes remain intact (blinking), laryngeal reflex. The respiratory center is not depressed and frequently there is an increase in heart rate and blood pressure. Ketamine hydrochloride is the commonly used dissociate anesthetic in veterinary medicine. In some species, such as cats and dogs, side effects of ketamine anesthesia include: excessive salivation, muscle rigidity, a tendency toward convulsions, and recovery characterized by excitement, disorientation, and hallucinations. The extent of analgesia provided for invasive procedures is questionable and Ketamine should not be used alone in these cases. The use of narcotic preanesthetics is recommended for the reduction of the side effects of ketamine. Telazol is a combination injectable anesthetic composed of tiletamine, a dissociative anesthetic related to ketamine, and zolazepam, a water soluble benzodiazepine tranquilizer. It is suitable for short noninvasive procedures in some species including primates and swine, and may be used for injection prior to general gas anesthesia. Tribromoethanol (Avertin) Tribromoethanol (Avertin) is an anesthetic that provides rapid induction and recovery for single use, short duration (approximately 15-20 minutes) surgical procedures in rodents. Tribromoethanol has been commonly used in the production of transgenic animals to facilitate procedures such as embryo transfer, vasectomy, or distal tail amputation for Southern Blot analysis. Tribromoethanol is not commercially available and investigators who wish to use it as an anesthetic must make their own solutions. Tribromoethanol may be approved for use after scientific and/or medical justification has been provided in the animal care and use protocol as to why a pharmaceutical grade anesthetic agent cannot be used. TJU strongly encourages the use of ketamine/xylazine or isoflurane rather than Tribromoethanol. Inhalation Anesthesia The use of inhalant anesthesia provides the user with several advantages over injectable anesthesia. The rapid induction and recovery as well as the overall controllability of the anesthesia provide a greater means of absolute control of the animal's physiological state. However, inhalant anesthesia requires specialized equipment, constant monitoring, and trained technical assistance. Equipment can be a simplistic nose cone for administration to rodents or complex apparatus required for larger species. Several devices have been designed for the administration of inhalant anesthesia to the variety of species commonly used in the laboratory and consultation of the literature may be helpful. Commonly used inhalant anesthetics are: Isoflurane is an anesthetic with almost "ideal" properties. It is non-flammable, non-irritating, non-toxic, and relatively insoluble in the blood. It has rapid induction and recovery. Isoflurane is encouraged as the first choice anesthetic in mice. It should be delivered as a known percentage (1-3% for maintenance; up to 5% for induction) in oxygen from a precision vaporizer. Sevoflurane is an inhalation anesthetic similar to isoflurane, but has a very rapid anesthesia induction and recovery. Methoxyflurane is a potent anesthetic which provides significant post-operative analgesia. Its low volatility allows its use with a simple nose cone for small rodents. Muscle relaxation is good and it is neither explosive nor irritating in anesthetic doses. Renal and hepatic damage is possible with prolonged or excessive doses. Halothane is a widely used inhalant anesthetic in veterinary medicine. It provides rapid induction and recovery, fair muscle relaxation, and poor analgesia. Halothane is highly volatile and should be used with a finely-calibrated precision vaporizer. It may induce malignant hyperthermia in certain breeds of swine. Nitrous Oxide is a weak anesthetic drug for veterinary use and must not be used by itself. It can be used both with other inhalant anesthetics or narcotic analgesia. Used in combination with other inhalant anesthetics it reduces the dose needed of these agents. Diethyl Ether is a highly volatile anesthetic agent. Ether is not an approved anesthetic agent at Thomas Jefferson University. A precision vaporizer must be used for delivering inhalant anesthetics. Use of a cotton ball or gauze soaked with the anesthetic in a closed container is strongly discouraged and can only be used if in an approved protocol. Strong justification will be required before approval is granted. Anticholinergic Drugs The use of anticholinergic drugs such as atropine or glycopyrrolate reduce salivation, lacrimation, urination, and defecation during the experimental procedure. Atropine is the most commonly used anticholinergic. It should be noted that rabbits have the enzyme atropinesterase which results in a decreased response to the administration of atropine. GUIDELINES FOR ANESTHESIA, ANALGESIA, AND TRANQUILIZATION o Cats o Dogs o Rats o Mice o Hamsters o Gerbils o Rabbits o Guinea Pigs o Primates o Pigs o Sheep & Goats Guidelines for Anesthesia, Analgesia, and Tranquilization: Tables CAT DRUG Acepromazine (tranquilization) Atropine Buprenorphine ANESTHESIA Premeds 0.03-0.05 mg/kg IV, IM, SC (2 mg max). 0.02-0.04 mg/kg IV, IM, SC 0.01 - 0.02 mg/kg IV, IM, SC ANALGESIA Induction Agent … … … … 0.005-0.01 mg/kg IV, IM, SC q6-12h … Buprenorphine SR … 0.12 mg/kg SC q72h … Butorphanol 0.2 - 0.4 mg/kg IV, IM, SC 0.2 – 0.4 mg/kg IM, SC q2-4h; tablets: 0.4 mg/kq q8h … Diazepam (tranquilization) 0.1-0.2 mg/kg IV … … … **1.25-2.5 mg patch (12.5-25 mcg/hr); duration at least 72 hrs and is longer in cats than dogs. … … … 0.1-0.2 mg/kg IV, IM, SC q 2-6h … … …. Fentanyl Glycopyrrolate Hydromorphone Isoflurane 0.01-0.02 mg/kg IV, IM, SC 0.1-0.2 mg/kg IV, IM, SC 1-3% inhalant to effect (up to 5% for induction) Ketamine 22-33 mg/kg can be used for dx or mionor sx procedures, rec. using with xylazine or diazepam … … Ketamine + Diazepam … … K: 5-10 mg/kg IV D: 0.05 mg/kg IV Meloxicam (NSAID) Meperidine Midazolam (tranquilization) … 2-6 mg/kg IV, IM, SC 0.06-0.2 mg/kg with opioid IV, IM, SC Morphine … Oxymorphone … 0.2 mg/kg PO, SQ loading dose once, followed by 0.1 mg/kg PO, SQ q 24h for not longer than 2 days. 3-5 mg/kg IM, SC q12h … … … … 0.1 mg/kg SC q1224h 0.05-0.1 mg/kg SC q1-3h … … … 1-2mg/kg PO q8-12h … … … 4-8 mg/kg IV … 1-4 mg IV, 4-8 mg IM … 8-10 mg/kg IV … … 0.5-2 mg/kg IV, IM, Xylazine … … SQ ** Small cats may be dosed with ½ patch, but the patch should not be cut in half. Cover ½ of the gel membrane with tape. “Half-patch dosing” is suggested for pediatric, geriatric, and systemically ill cats. Propofol Telazol Thiopental Tramadol DOG ANESTHESIA Premeds DRUG Aspirin Acepromazine (tranquilization) Atropine Buprenorphine ... 0.03-0.05 mg/kg IV, IM, SC (2 mg max). 0.02-0.04 mg/kg IV, IM, SQ 0.01 - 0.02 mg/kg IV, IM, SC Buprenorphine SR … Butorphanol 0.2 - 0.4 mg/kg IV, IM, SC ANALGESIA Induction Agent 10-20 mg/kg q12h … … … … … 0.005-0.02 mg/kg IV, IM, SC q6-12h 0.03-0.06 mg/kg SC q 72h 0.2 – 1 mg/kg IM, SC q2-4h; tablets: 0.4 mg/kq q8h … … … Carprofen (NSAID) … 2 mg/kg PO q12h … Codeine … 0.5-2 mg/kg PO q612h … Deracoxib (NSAID) Diazepam (tranquilization) … 1-2 mg/kg PO q24h … 0.1-0.2 mg/kg IV … … Fentanyl … See below … 0.01-0.02 mg/kg IV, IM, SC 0.1-0.2 mg/kg IV, IM, SC 1-3% inhalant to effect (up to 5% for induction) D: 0.5 mg/kg IV then ketamine K: 10 mg/kg IV … … 0.1-0.2 mg/kg IV, IM, SC q 2-6h … … … … … Glycopyrrolate Hydromorphone Isoflurane Ketamine + Diazepam Meloxicam (NSAID) Meperidine Midazolam (tranquilization) … 2-6 mg/kg IV, IM, SC 0.06-0.2 mg/kg with opioid IV, IM, SC Morphine 0.1-2 mg/kg IM, SC Oxymorphone … Pentobarbital Propofol Thiopental Tramadol 30 mg/kg IV … … … 0.5-2 mg/kg IV, IM, SC Xylazine 0.2 mg/kg PO, SQ loading dose once, followed by 0.1 mg/kg PO, SC q 24h 3-5 mg/kg IM, SC q1-2h … 0.5-2 mg/kg IM, SC q3-4h; tablets: 1.5-3 mg/kg PO q12h 0.22 mg/kg SC q 13h … … … 1-5 mg/kg PO q8-12h … … … … … … … 4-8 mg/kg IV 8-10 mg/kg IV … … DOG Fentanyl Canine Size Dose Fentanyl Content < 5 kg ** 12.5- 25 mcg/hr 1.25-2.5 mg 5-10 kg 25 mcg/hr 2.5 mg 10-20 kg 50 mcg/hr 5 mg 20-30 kg 75 mcg/hr 7.5 mg > 30 kg 100 mcg/hr 10 mg ** Small dogs may be dosed with ½ patch, but the patch should not be cut in half. Cover ½ of the gel membrane with tape. “Half-patch dosing” is suggested for pediatric, geriatric, and systemically ill dogs. RAT & MOUSE: Ketamine, Acepromazine, and Xylazine cocktail ± Atropine. It is strongly recommended that atropine be administered (as a separate injection) due to the cardiovascular depressant effects of the following mixture. In one bottle of ketamine (100 mg/ml) add two ml of acepromazine (10 mg/ml) and 0.5 ml of xylazine (100 mg/ml). * Please note that the 100 mg/ml bottle of xylazine is used, not the 20 mg/ml bottle. This will give final concentrations of 80 mg/ml of ketamine, 1.6 mg/ml of acepromazine, and 5 mg/ml of xylazine. Dose the animal at 1.25 mls/kg of body weight or 0.125 mls/100 grams of body weight subcutaneously (works nicely using the SC route, can probably also be administered IP). Atropine: Given at approximately 0.05 mg/kg mix 0.25 mls of atropine in 4.75 mls of normal saline. This will give a final concentration of 0.027 mg/ml. Dose the animal at 0.054 mg/kg or 0.2 mls/100 grams of body weight subcutaneously. The recommended shelf life for the ketamine, xylazine, aceproazine cocktail is 180 days after mixing, provided that any of the 3 drugs in the mixture have not expired before that time. Taylor, B. et al. 2009. Beyond-Use Dating of Extemporaneously Compounded Ketamine, Acepromazine, and Xylazine: Safety, Stability, and Efficacy Over Time. JAALAS 48(6): 718-726. Atropine in saline should be discarded immediately after use. Rat & Mice Tribromoethanol (Avertin) Improper preparation, storage, or use of Tribromoethanol can result in high mortality losses. In particular, Tribromoethanol degrades in the presence of heat and light, producing toxic by-products that are potent gastrointestinal irritants. The procedure for production, storage and use of Avertin was provided by the Jackson Laboratory, where it is used routinely for mouse surgery. Preparation of Tribromoethanol Stock Solution Items Needed: 1ml syringes 10ml syringes 100ml wide mouth glass bottle (2) amber vials with silicone septa, 40ml 50g 2,2,2 tribromoethanol powder tert-amyl alcohol 25mm syringe filter with 45um membrane pH paper (range 4.0 to 7.0) Tribromoethanol stock labels Fisherbrand® marking pen Clean lab coat Gloves NOTE: Glass bottle and amber vials must be cleaned and steam sterilized prior to use. Preparation of stock solution 1. In a fume hood add 31mls tert-amyl alcohol to 50g of 2,2,2tribromoethanol powder in a 100ml wide mouth glass bottle. Cap the bottle tightly and manually shake for a few minutes. This should get most of the powder into solution. 2. Place the bottle in a 600C shaker water bath. Gently shake the bottle until all of the crystals are dissolved. 3. Once dissolved test the pH of the stock solution. It should be between 5.0 and 5.5. 4. In a laminar flow clean bench use 10ml syringes to withdraw the tribromoethanol stock solution from the bottle. 5. Attach a syringe filter to a 10ml syringe and filter the tribromoethanol stock solution directly into the 40ml amber vial. Use a new syringe and syringe filter for each 10mls of stock solution. 6. Repeat Steps 4-5 until all the stock solution is removed from the bottle. 7. Place a stock label on the bottle. Record on the label: chemical name, preparation date, expiration date, who prepared the stock solution, and bottle number. NOTE: There is 1-month expiration date for tribromoethanol stock solution. 8. Store stock solution in dark at room temperature. Preparation of the working solution of tribromoethanol (20mg/ml) Items Needed: Amber vials with silicone septa, filled with 39.5mls 1X PBS Tribromoethanol stock solution 1ml syringes 20 gauge needles 70% ethanol pH paper (range 4.0 to 7.0) Fisherbrand® marking pen Clean lab coat Gloves NOTE: Amber vials must be cleaned and steam sterilized prior to use. Preparation of working solution 1. Place the vials containing PBS into a water bath, bring up to 500C and heat for 10 minutes. 2. In a laminar flow clean bench fill 1ml syringes with tribromoethanol stock solution. 3. After 10 minutes at 500C remove and dry vials and place in the laminar flow clean bench. 4. Disinfect the top of each vial with 70% ethanol. 5. Inject 0.5ml of stock solution into each vial while the PBS is still warm and manually shake each vial until the solution is thoroughly mixed (no bubbles/crystals visible). 6. Test the pH of one vial in the batch. The pH should be 7.0. 9. Place a 2% tribromoethanol label on the bottle. Record on the label: chemical name, preparation date, expiration date, who prepared the stock solution, and bottle number. NOTE: There is a 3-week expiration date for tribromoethanol working solution. 10. Store working solution in dark at room temperature. Dosage: The dosage for the C57BL/6J mouse is ~0.2 ml/10 gram body weight (400 mg/kg) IP. Dosage will need to be adjusted by mouse strain. RATS DRUG Acepromazine ANESTHESIA … ANALGESIA … 200 - 300 mg/kg PO 1-2 mg/ml in drinking water 100-150 mg/kg PO q4h … 0.1-0.5 mg/kg SC, IP q8-12h 0.9-1.2 mg/kg SC q72h 0.2-2 mg/kg SC, IP q2-4h … 1.1-2.5 mg/kg SC q12h TRANQUILIZATION 0.5-1 mg/ kg IP, SC Acetaminophen (NSAID) … Aspirin (NSAID) … Atropine (premed) 0.1-0.4 mg/kg SC Buprenorphine … Buprenorphine SR … Butorphanol … Diazepam … Flunixin (NSAID) … Glycopyrrolate (premed) 0.01-0.02 mg/kg SC … … Ibuprofen (NSAID) … 10-30 mg/kg PO q4h … … … … … … … Isoflurane Ketamine + acetylpromazine Ketamine + Diazepam 1-3% inhalant to effect (up to 5% for induction) K: 75 mg/kg IP, SC A: 2.5 mg/kg IP, SC K: 60 - 80 mg/kg IP, SC D: 10 mg/kg IP, SC … … … … … … 4.0 mg/kg SC … Ketamine + Xylazine + Acepromazine See description above. … … Ketamine + Xylazine K: 40 - 80 mg/kg IP, SC X: 5 - 10 mg/kg IP, SC … … Meloxicam (NSAID) … … Meperidine … Midazolam … Morphine … Oxymorphone … 0.2-0.3 mg/kg PO, SC q12h 10-20 mg/kg SC q2-3h … 2-5 mg/kg SC q2-4h 0.2-0.5 mg/kg SCq6-12h … 1-2 mg/kg SC … … Pentobarbital 30-45 mg/kg IP … … * Different strains and stocks may react differently to drug combinations. MICE DRUG ANESTHESIA ANALGESIA TRANQUILIZATION 0.5-1.0 mg/kg IP, SC … Acepromazine … … Atropine (premed) 0.05 - 0.1 mg/kg SC Acetaminophen (NSAID) … Aspirin (NSAID) … Buprenorphine .. Buprenorphine SR … Butorphanol … Carprofen (NSAID) Diazepam … … Flunixin (NSAID) … … 300 mg/kg PO 1-2 mg/ml in drinking water 120 mg/kg PO q 4h 0.05-2.5 mg/kg SC, IP q6-12h 0.3 0.6 mg/kg SC q72h 1-5 mg/kg SC q 4h 5mg/kg SC q24h … 0.3 -2 mg/kg SC, Po q12-24h Glycopyrrolate (premed) 0.01-0.02 mg/kg SC … … Ibuprofen (NSAID) .. 7-15 mg/kg PO q4hr … … … … … … … … … … … 3-5 mg/kg IP … Ketamein + Xylazine 1-3% inhalant to effect (up to 5% for induction) K: 50 mg/kg IP, SC X: 5 mg/kg IP, SC Meloxicam (NSAID) … 0.2-0.3 mg/kg PO, SQ q 12hrs … Ketamine + Xylazine + Acepromazine See description above. … … Meperidine … … Morphine … Oxymorphone .. Pentobarbital 50-90 mg/kg IP, SC 20 mg/kg Sc q23h 2-5 mg/kg SC q2-4h 0.2-0.5 mg/kg SC q6-12h … Isoflurane … … … * Different strains and stocks may react differently to drug combinations. HAMSTERS DRUG ANESTHESIA ANALGESIA TRANQUILIZATION 0.5-1.0 mg/kg IP, SC Acepromazine … … Acetaminophen (NSAID) Atropine (premed) … Buprenorphine … Butorphanol … 1-2 mg/ml in water ... 0.5 mg/kg SC q8h 1-5 mg/kg SC q4h Carporfen (NSAID) Diazepam … 5 mg/kg SC q24h … … 3-5 mg/kg IP Flunixin (NSAID) … … 2.5 mg/kg SC q12-24h Glycopyrrolate (premed) 0.01-0.02 mg/kg SC … … 1-3% inhalant to effect (up to 5% for induction) K: 80 mg/kg IP X: 5 mg/kg IP … … … … … 0.2-0.3 mg/kg PO, SC q12h 20 mg/kg SC q23h 2-5 mg/kg SC q8h 0.2-0.5 mg/kg SC q6-12h … … Isoflurane Ketamine + Xylazine Meloxicam (NSAID) 0.05-0.1 mg/kg SC Meperidine … Morphine … Oxymorphone … Pentobarbital 50-90 mg/kg IP ... … … … … … … … … Hamsters exhibit a great variability of response to administration of parenteral anesthesics. The use of droperidol and fentanyl may result in CNS signs and should be avoided if possible. Intravenous injections may be made into the saphenous vein. GERBILS DRUG Acetaminophen (NSAID) Atropine (premed) ANESTHESIA … Buprenorphine … Butorphanol … 0.05-0.1 mg/kg SC ANALGESIA 1-2 mg/ml in water … 0.1-0.2 mg/kg SC q8h 1-5 mg/kg SC q4h TRANQUILIZATION … … … Carprofen (NSAID) Diazepam … 5 mg/kg SC q24h … … 3-5 mg/kg IP Flunixin (NSAID) … ... 2.5 mg/kg SC q12-24h Glycopyrrolate (premed) 0.01-0.02 mg/kg SQ … … 1-3% inhalant to effect (up to 5% for induction) K: 50 mg/kg IP, SC X: 2 mg/kg IP, SC … … … … … 0.2-0.3 mg/kg PO, SC q12h 20 mg/kg SC q23h 2-5 mg/kg SC q8h 0.2-0.5 mg/kg SC q6-12h … … Isoflurane Ketamine + Xylazine Meloxicam (NSAID) Meperidine … Morphine … Oxymorphone … Pentobarbital 50-90 mg/kg IP … … … … … In gerbils Acepromazine can cause seizures. RABBITS DRUG Acepromazine … Aspirin (NSAID) … Buprenorphine … Buprenorphine SR … Butorphanol … Carporfen (NSAID) … Diazepam … Fentanyl … Flunixin (NSAID) … Glycopyrrolate (premed) 0.01-0.02 mg/kg SC … … 1-3% inhalant to effect (up to 5% for induction) K: 25-40 mg/kg IM, SC A: 0.25-1 mg/kg IM, SC … … … … Isoflurane Ketamine + Acepromazine ANESTHESIA ANALGESIA … 100 mg/kg PO q24h 0.02-0.1 mg/kg SC q6-12h 0.1-0.3 mg/kg SC q72h 0.1-0.5 mg/kg IM, SQ q4h 1-2 mg/kg POq12h; 2-4 mg/kg PO q24h … 2.5 mg patch (25 mcg/hr) x 3 days 1-2 mg/kg SC q1224h TRANQUILIZATION 1-2 mg/kg SQ, IM … … … … … 1-5 mg/kg IM … … Ketamine + Diazepam Ketamine + Xylazine Meloxicam (NSAID) K: 30-40 mg/kg IM, SC D: 2-5 mg/kg IM, SC K: 30-40 mg/kg IM, SC X: 5 mg/kg IM, SC … … … … … … Meperidine … Midazolam … 0.2-0.3 mg/kg PO, SC q12-24h 5-10 mg/kg SC, IM q2-3h … Morphine … … Pentobarbital 20 - 45 mg/kg IV (pre anesthetize with 1 mg/kg Ace IM … … 1-2 mg/kg IM 2-5 mg/kg IM, SC q4h … Intramuscular injections of ketamine have been associated with local necrosis and distal neuropathies in the injected leg. GUINEA PIGS DRUG Acepromazine Aspirin (NSAID) Atropine (premed) ... ... ANESTHESIA ANALGESIA ... 80-85mg/kg PO q4h TRANQUILIZATION 0.5-1 mg/kg IM ... 0.1-0.2 mg/kg SC, IM … … Buprenorphine ... Butorphanol … Carprofen (NSAID) … Diazepam Glycopyrrolate (premed) Ibuprofen ... 0.01-0.02 mg/kg IM, SQ ... 1-3% inhalant to effect (up to 5% for induction) K: 20-40 mg/kg A: 0.5 mg/kg IM K: 20-30 mg/kg IM D: 1-2 mg/kg IM K: 20-40 mg/kg IM X: 2 mg/kg IM Isoflurane Ketamine + Acepromazine Ketamine + Diazepam Ketamine + Xylazine Meloxicam (NSAID) … 0.05-0.10 mg/kg SC q6-12h 0.4-2 mg/kg SC q24h 1-2 mg/kg PO q1224h; 4 mg/kg SC q24h ... ... … … 0.5-3 mg/kg IM … … 10 mg/kg PO q4h ... ... ... ... ... … … ... … 0.2-0.3 mg/kg PO q12h … Meperidine ... Morphine ... 10-20 mg/kg SC,IM q2-3h 5-10 mg/kg SC,IM q4h ... ... Guinea pigs are an anesthetic challenge. They lack accessible blood vessels for IV administration and display great variability in response. It is not uncommon for two guinea pigs of the same sex, age, weight, and strain to respond quite differently to the same anesthesia. Guinea pigs can also exhibit a swimming motion with their legs during the surgical plane of anesthesia. Halothane is considered high risk for guinea pigs as they hold their breath and then gasp. Halothane (1%) causes a 35% - 40% drop in blood pressure. Isoflurane has been used with great success in guinea pigs. Innovar Vet® if injected IM can cause necrosis and sloughing of the skin at the injection site. PRIMATES DRUG Acepromazine Malate ANESTHESIA ANALGESIA TRANQUILIZATION … … 0.5 - 1 mg/kg IM, SC Aspirin (NSAID) … 100 mg/kg PO q24h … Atropine 0.02-0.05 mg/kg SC, IM, IV … … Buprenorphine … … Buprenorphine SR … Butorphanol … 0.01 - 0.03 mg/kg IM q6-12h 0.2 mg/kg SC q5 days Old World Primates: 0.5 mg/kg IM q8h New World Primates: 0.02 mg/kg SQ q6h Carprofen (NSAID) … 2 mg/kg PO q12h … Diazepam … … 0.25-0.5 mg/kg IM, IV Flunixin (NSAID) … … Glycopyrrolate Ibuprofen (NSAID) 0.005-0.01 mg/kg IM 10 mg/kg IM q1224h … … 7 mg/kg PO q12h … 1-3% inhalant to effect (up to 5% for induction) … … Isoflurane … … … Ketamine Ketamine + Acepromazine Ketamine + Diazepam Ketamine + Dexmedetomidine Ketamine + Xylazine 5 - 10 mg/kg IM K: 4 mg/kg IM A: 0.04 mg/kg IM K: 15 mg/kg IM D: 1 mg/kg IM K: 3-5 mg/kg IM D: 0.01-0.03 mg/kg IM K: 7 mg/kg IM X: 0.5 mg/kg IM Meloxicam (NSAID) … Oxymorphone … Propofol (induction) Telazol … … … … … … … … … … 0.1-0.2 mg/kg PO q24h for up to 3 days Old world: 0.15 mg/kg SC, IM q4-6h New world: 0.075 mg/kg SC, IM q4-6h … … 2.5 - 5.0 mg/kg IV … … 2 - 6 mg/kg IM … … PIG ANESTHESIA Premeds DRUG ANALGESIA TRANQUILIZATION Acepromazine Aspirin (NSAID) Atropine … …. 0.1 - 0.22 mg/kg IM … 10 mg/kg PO q12h … 0.04 mg/kg IM … Buprenorphine … Butorphanol … … 0.005 - 0.1 mg/kg IM q8-12h; use 0.05-0.1 mg/kg for major surgeries 0.1 - 0.3 mg/kg IM q812h Carprofen (NSAID) Diazepam Fentanyl … 1 - 2 mg/kg PO q12h … … … 1-3% inhalant to effect (up to 5% for induction) … Use Dog Dose 0.5 - 10 mg/kg IM … … … K: 12-20 mg/kg IM X: 1-2 mg/kg IM … … 2 - 10 mg/kg IM q4h Isoflurane Ketamine + Xylazine Meperidine … … … … Morphine Propofol (induction) Telazol … 0.2 - 0.9 mg/kg SC, IM q4h … 4-8 mg/kg IV … … 3-5 mg/kg IM … … SHEEP & GOATS DRUG Acepromazine ANESTHESIA … Buprenorphine … Butorphanol … Diazepam … Flunixen (NSAID) Isoflurane Pentobarbital Propofol (induction) to effect 20 - 30 mg/kg IV Thiopental Ketamine Xylazine ANALGESIA TRANQUILIZATION 0.05 - 0.1 mg/kg IM … Sheep: 0.015-0.01 mg/kg IM, SQ q612h … Goats: 0.005 mg/kg IM, SC q6-12h 0.2 – 1 mg/kg IM, SC … q2-4h Sheep: 1.0 - 1.5 mg/kg IM; 5 mg/kg in feed … Goats: 0.5 - 1.5 mg/kg IM; 15 mg/kg in feed 0.5-1 mg/kg PO, IM, IV q8hrs … … … … 2-4 mg/kg IV Sheep: 10-15 mg/kg IV Goats:20 - 22 mg/kg IV K: 15 mg/kg IM, X:, 0.1-0.2 mg/kg IM … ... … … Reversal Agents Atipamezole (Antisedan) Yohimbine (Yobine) Reverses Effects of Medetomidine (Domitor) Xylazine (Rompun) Non-rodent species Give IM an equal vol of Antisedan as Domitor. May give IV if it has been 45 min since Domitor was given. May give at equal volume or ½ volume of Domitor dose. Rabbits Other 1 mcg/kg SC, IV Mice, Rats, Gerbils, Hamsters, Guinea Pigs Dogs, Cats, Primates: 0.100.15 mg/kg IV slowly. 0.2-1 mg/kg IM, IV 0.1 – 0.2 mg/kg IM, SC Sheep & Goats: 0.13-0.22 IV slowly. Pigs: 0.125-0.3 mg/kg IV slowly. 0.1-1 mg/kg IM, SC, IV, IP Mice, Rats, Gerbils, Hamsters, Guinea Pigs: 0.2-0.5 mg/kg IP Local Analgesia for Rodents Purpose: To provide additional pain relief directly at the site of surgery before making the surgical incision (i.e., incisional line block). Local anesthesia can be used in addition to systemic analgesia such as opioids or NSAIDs to control pain. Local analgesic drugs should not be used as the sole analgesia for moderate to severe pain. Route: Local anesthetics are given subcutaneously. Intramuscular and Intravenous injections must be avoided. Systemic toxicity (seizures, heart rhythm disturbances, and death) results from over dosage or accidental intravenous injections. Local Anesthetic Lidocaine (2%) (20 mg/ml) Dose Dilute to 0.5% (5 mg/ml), do not exceed 7 mg/kg total dose Without epinephrine Bupivacaine/Marcaine Dilute to 0.25% (2.5 (0.5%) mg/ml), do not exceed 8 mg/kg total dose Without epinephrine Ropivacaine/Naropin (0.2%) Maximum Volumes: Weight of Mouse 25 g 35 g 45 g 55 g Weight of Rat 250 g 350 g 450 g 550 g No need to dilute, do not exceed 8 mg/kg total dose. Dilutions 1:4 dilution - Take 1 ml of lidocaine (2%) and add 3 ml of sterile water. Discard after using. 1:2 dilution – Take 1 ml of 0.5% bupivacaine and add 1 ml of sterile water. Discard after using. N/A Notes Faster onset than bupivacaine but short (< 1 hr) duration of action Slower onset than lidocaine but longer (4-8 hr) duration of action Slower onset than lidocaine but longer (4-8 hr) duration of action; wider safety margin Maximum Volume Diluted Lidocaine (0.5%) Do Not Exceed 0.03 ml 0.05 ml 0.06 ml 0.07 ml Maximum Volume Diluted Bupivacaine (0.25%) Do Not Exceed 0.08 ml 0.11 ml 0.14 ml 0.17 ml Maximum Volume Ropivacaine (0.2%) Maximum Volume Diluted Lidocaine (0.5%) Do Not Exceed 0.35 ml 0.49 ml 0.63 ml 0.77 ml Maximum Volume Diluted Bupivacaine (0.25%) Do Not Exceed 0.8 ml 1.12 ml 1.44 ml 1.76 ml Maximum Volume Ropivacaine (0.2%) Do Not Exceed 0.1 ml 0.14 ml 0.18 ml 0.22 ml Do Not Exceed 1.0 ml 1.4 ml 1.8 ml 2.2 ml Use of Hypothermia as Anesthesia for Neonatal Rodents Hypothermia may be used to induce anesthesia in neonatal rats and mice less than six days of age. Their small size and body mass makes rapid core cooling feasible through surface cooling. They are also more resistant to arrest of blood supply to the brain and tolerate extended periods of 1°C body temperature without known negative effects. Potential risks of hypothermia include ventricular fibrillation, tissue hypoxia and metabolic acidosis on warming. When a need for hypothermia is demonstrated, the following guidelines should be followed: To induce hypothermia, pups may be placed in a latex sleeve or glove and immersed up to the neck in crushed ice and water (2°C-3°C) which requires a 5-8 minute induction time (2-3 minutes to unconsciousness and 3-5 minutes to complete blockage of neural transmission). Alternatively, pups may be placed in a paper-lined tube and packed in crushed ice which may require up to 15 minutes to obtain a surgical plane of anesthesia. Analgesia for hypothermia induced by these methods lasts approximately 10 minutes. Do not place the animals directly on the cooling medium; provide a cloth, paper, or other barrier material. Simply placing conscious animals in a cold room or on an ice pack are not acceptable as induction may take 30-45 minutes. The anesthetic state may be prolonged by placing the hypothermic pup on an ice pack (3°C-4°C). Studies have shown that rodent pups will maintain a core body temperature of approximately 5°C when kept on an ice pack for a maximum of 15 minutes. Illumination of the surgical field should be fiber optic in nature, because incandescent bulbs may cause inadvertent and uncontrollable warming. After the procedure, avoid rapid re-warming because of possible tissue damage. Until animals are fully conscious they cannot ‘escape' excessive heat generated by incandescent lamps, heating pads, or other warming devices. Pups can also be placed in an incubator at 33°C for 20-30 minutes. Complete recovery typically requires 30-60 minutes. Supplemental oxygen may benefit the recovering animals.