Specimen types
advertisement

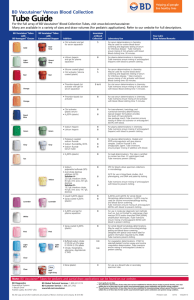
Overview of Specimen Types The three types of specimens most often collected in studies are blood, stool, and urine. Blood: Blood can be taken from the vein (most common), artery (blood gases only), the finger, or heel on babies. Venous blood (blood from the arm vein) gives you the most volume and is easy to work with. Venipuncture is the preferred method of collecting blood if a number of tests are going to be done. Finger sticks (on adults or children >12 months of age) are good for tests that require a small amount of blood (e.g., hemoglobin). If more blood is needed from the finger, it can be collected into what is commonly referred to as a Microtainer. These may contain EDTA (to keep the blood from clotting) or have no additive to allow the blood to clot. Brown Microtainers are also available, so that light does not adversely affect photo labile analytes (i.e., vitamin A). If blood is collected in a purple top tube that contains EDTA as an anticoagulant, the blood will not clot. This blood can be used for cell counts, malaria smears, hemoglobin analysis, and many other tests that require un-clotted whole blood. The different colored tops indicate what the tube can be used for as well as what kind of anticoagulant is or is not in the tube. Anticoagulants that are used with other types of tests are ACD in yellow top tubes, heparin in green top tubes, and potassium oxalate and sodium fluoride in gray top tubes. There are also some other tubes that are used in genetic studies that have a blue/black top (or some other unique color). If blood is collected in a tube with an anticoagulant and it is centrifuged, the fluid at the top is called plasma. Plasma has a tendency to form micro clots when sitting frozen or refrigerated that can cause major problems when trying to pipette these samples. That is one reason why serum is preferred over plasma. If blood is collected in a tube that does not contain EDTA or an anticoagulant, this blood will begin to clot immediately. The blood will completely clot and be ready to centrifuge in 30-90 minutes. This blood is usually drawn in a red top tube. There are also tubes that allow the blood to clot which have a gel in the bottom, and are known as serum separator or gel tubes. These come in a variety of colors, but the two most commonly used are a gold top tube and a black/red “speckled” tube. If blood is collected in a clot tube and centrifuged, the fluid on top is known as serum. Serum has all of the clotting factors used up and is the opposite of plasma in that regard. Generally, you cannot substitute plasma for serum unless the test you are doing allows this. There is some difference in the results of most tests using serum instead of plasma. You should not centrifuge any clot tube until the blood has completely clotted, which takes a minimum of 30-90 minutes. If you allow the blood to sit longer than this and you then centrifuge it, some of the analytes you are testing for may be elevated or decreased due to the excess clotting time. It is important to try and standardize your clotting time prior to centrifugation to minimize effects caused by allowing the blood to clot for an extended period. Gel tubes need a certain g-force in order for the gel to migrate up the tube during the centrifugation process. Generally, clot tubes are spun for a minimum of 10 minutes at approximately 2,500 rpm. This speed can change based on the centrifuge, the manufacturer’s instructions, and the type or size of tube being centrifuged. The word Vacutainer is a brand name that Becton Dickinson uses for all their blood drawing tubes. These tubes have a vacuum in them causing them to act like a syringe when you penetrate the rubber top with a multi-sampler needle. In the old days, only syringes were used to draw blood from veins and arteries. Glass syringes were used in the "very old" days and plastic is now the norm. Tubes and vials are different. The word “vial” usually indicates a small (~2 mL) plastic container with a screw cap or snap cap. Tubes, on the other hand, are larger and hold a much larger amount of blood (~3-10 mL). Important things to remember when collecting EDTA or anticoagulated blood: 1. The tube must be mixed well as soon as possible either during collection or immediately after. You should mix the tube 8-10 times by inverting the tube completely. Do not shake the tube as this may cause hemolysis and foaming. The word hemolysis indicates that the red cells have been lysed and any cell morphology is useless. Hemolysis can also interfere with certain clinical chemistry tests. Hemolysis is indicated by the serum or plasma being a reddish color instead of a light yellow. 2. Clotting of any anticoagulated blood renders it useless, and it should be discarded. Another sample should be collected in a proper manner, so that it does not clot. This is especially a problem with finger sticks if you take too long to collect the specimen or you do not mix it well. 3. The EDTA tube needs a specific amount of blood or the specimen will be over diluted by the anticoagulant. Usually, if you can fill the tube more than half way, that would be the minimum amount. Ideally, you want to fill the tube to the prescribed volume designated for the particular EDTA tube. This is true for any anticoagulated tube. 4. Storage of the sample collected will depend on the test(s) being done on the blood in that tube. Stool: The collection and handling of stool can best be described by the staff handling the parasitology portion of the study. Urine: Urine can be collected “on the spot” for random testing, or the urine can be collected over a 24 hour period into a large container and brought in for testing. This will vary from survey to survey. Storage of the urine will be based on the type of testing that will be done. Dipstick testing should be done as soon as possible on freshly collected urine. Use of trade names and commercial sources is for identification only and does not imply an endorsement by the U.S. Department of Health and Human Services (DHHS) and the Centers for Disease Control and Prevention (CDC).