ELISA as a Diagnostic Tool — Influenza - Bio-Rad
advertisement

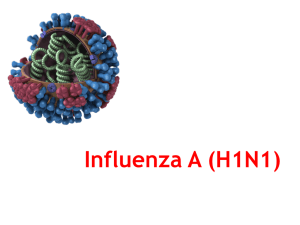
ELISA Immuno ExplorerTM Influenza Diagnostic Tool ELISA Immuno ExplorerTM Kit Influenza Diagnostic Tool Instructors Stan Hitomi Coordinator – Math & Science Principal – Alamo School San Ramon Valley Unified School District Danville, CA Kirk Brown Lead Instructor, Edward Teller Education Center Science Chair, Tracy High School and Delta College, Tracy, CA Bio-Rad Curriculum and Training Specialists: Sherri Andrews, Ph.D. sherri_andrews@bio-rad.com Leigh Brown, M.A. leigh_brown@bio-rad.com Why Teach ELISA? • Hands-on Immunology • Tangible results • Laboratory extensions • Real-world connections • Link to careers and industry • Standards-based: One lesson integrates multiple standards –Health sciences –Immunology –Immune response – antibody/antigen interactions –Disease – infection, detection, transmission ELISA Immuno Explorer Kit Advantages • Lab completed in a 45 min period • Supplies for 48 students (12 workstations) • Comprehensive and flexible curriculum • Compelling real-world links • Striking results • Cost effective • Classroom Safe Workshop Time Line • Introduction • Rapid Influenza Diagnostic Test (RIDT) • Viruses, influenza, and H1N1 • Ways the ELISA Immuno Explorer Kit can be used Lab Scenario • A room full of sick people (you guys!) • Various symptoms – Coughing – Sneezing – Temperature – Other nasties! (what are you doing here, anyway?) Question: • Is this the 2009-2010 pandemic H1N1? • Food poisoning? • Cholera? • Or lots of psychosomatic symptoms (because the person next to you is sick)? Solution: Perform Rapid Influenza Diagnostic Test (RIDT) • RIDT is an ELISA that can be performed in the doctor’s office in less than 30 minutes • There are 3 RIDTs currently approved for use in the U.S. ELISA Immunoglobulin (IgG) Structure Enzyme-Linked Immunosorbant Assay Heavy chain Light chain Disulfide bonds Influenza Antigens RIDT detects viral antigens 1) Load samples & controls into wells Wash 2) Add primary antibody to all wells Wash 3) Add enzymelinked secondary antibody to all wells Wash 4) Add enzyme substrate to all wells ELISA ANIMATION Laboratory Quick Guide For Protocol II Steps 1 – 2 Label wells of microplate strip • Obtain a microplate strip and “serum samples” • Label the 12-well strip –First 3 wells: positive controls “+” –Next 3 wells: negative controls “-” –Remaining wells to identify test samples Sample 1 Sample 2 Steps 3 – 6 Add controls and samples • Add 50 µl of positive control to the 3 (+) wells • Using a fresh pipet tip, add 50 µl of negative control to the 3 (−) wells • Using a fresh pipet tip, add 50 µl of sample 1 to the next 3 wells • Using a fresh pipet tip, add 50 µl of sample 2 to the final 3 wells • Incubate for 5 minutes Microplate Strips • Microplate strips are made of polystyrene • Hydrophobic side chains of amino acids bind to the polystyrene wells • If flu antigen is present it will bind to the polystyrene, (+) control, and possibly in the unknown sample Influenza species (antigen types) 5 genera, but only 3 of interest to us Each genera has a single species! • Type A –Natural host: wild aquatic birds –Has serotypes (based on antibody response) • Type B –Infects mostly humans (ferrets & seals can get it too) –Less common than Type A –Mutation rate 2-3x slower than type A, so less genetic diversity and more acquired immunity • Type C –Infects humans, dogs, & pigs, but less common –Causes only mild disease Steps 7 – 8 Wash plates • Remove sample from wells by firmly tapping the strip on a paper towel • Discard the top paper towel • Using a disposable transfer pipet, wash wells with wash buffer • Remove wash buffer from wells by firmly tapping the strip on a paper towel • Discard the top paper towel • Repeat wash step Steps 9 – 10 Add primary antibody • Using a fresh pipet tip, add 50 µl of primary antibody to each well of the microplate strip • Incubate for 5 minutes • If any flu antigen bound to the well in previous step primary antibody will bind to antigen. Wash Buffer • Wash buffer contains phosphate buffer saline (PBS) to keep antibodies in a stable environment that helps keep their structure • Also contains Tween 20: a nonionic detergent that removes non-specifically bound proteins and coats wells to act as a blocking agent to reduce background • Antibody will bind only to influenza antigens Chemistry in action…. Or… Ask your friendly chemist… about detergents. DETERGENTS: … are amphiphiles, containing a lipophilic portion and a hydrophilic portion. lower the interfacial energy between unlike phases. emulsify or solubilize aggregated particles. I like fat! I like water! More about detergent terms Lipophilic portion is also referred to as “hydrophobic” tail Hydrophilic portion is also referred to as “polar” head Types: nonionic, anionic, cationic and zwitterionic Detergents: Ionic vs nonionic Denaturing vs non-denaturing Swords (denaturing): “pointy” hydrophobic ends, ionic polar ends Gloves (nondenaturing): bulky, nonpenetrating hydrophobic ends, non-ionic or zwitterionic polar ends. SDS Triton X-100 Steps 11 – 13 Wash & add enzyme-linked secondary antibody • Wash unbound primary antibody from microplate wells as before • Wash twice • Add 50 µl of the enzyme-linked secondary antibody to each well • Wait 5 minutes Antibody Specificity • Secondary antibody (enzyme-linked antibody) will only bind to the primary antibody • Secondary antibody specifically recognizes the constant region of the primary antibody Steps 14 – 15 Add enzyme substrate • Wash unbound enzyme-linked secondary antibody from microplate wells as before • Wash THREE times • Add 50 µl of the enzyme-linked substrate to each well • Wait 5 minutes • The positive samples will begin to turn blue Results • Some positive by RIDT • Some negative • Did the controls work? CDC guidelines for RIDTs (+) for Flu B (+) for Flu A (-) for Flu A & B Detect and distinguish between Type A and Type B influenza viruses OR Detect Type A and Type B influenza viruses, but not tell them apart OR Detect Type A influenza virus What about H1N1? • RIDT’s do not distinguish H1N1 specifically from other Type A Flu viruses. Lab tests for H1N1/09 • The most sensitive & specific laboratory tests are rRT-PCRs (real-time reverse transcriptase PCR) • rRT-PCRs detect viral RNA (very specific) • Cannot be performed in doctor’s office; 2-4 days to get results (test takes 6-8 hours) The flu! • Influenza viruses are single-stranded RNA viruses • Family Orthomyxoviridae • Affect birds and mammals • 3 types A, B, and C • 2009 H1N1 is Type A Influenza Type A • Roughly spherical virus, 80-120 nanometers • Viral envelope with 2 types of glycoprotein wrapped around central core • Core contains RNA genome and viral packaging proteins • Single-stranded (-)RNA virus; 8 RNA molecules encode 11 proteins Influenza A viral proteins • Hemagglutinen (HA)- viral glycoprotein that mediates binding of virus to target cell and entry of viral genome into that cell • Neuraminidase (NA)- viral glycoprotein that allows release of progeny virus from infected cells –H & N? Sound familiar? (think H1N1) • 16 HA subtypes – (H1-H16) • 9 NA subtypes (N1-N9) New human viruses • New human influenza viruses occur through: –Genetic reassortment within an existing human virus –Avian viruses developing capacity for human-to-human transmission • New influenza viruses may have novel HA proteins, with or without a novel NA proteins • Called antigenic shift • Novel antigens means that humans have no prior immunity 2009 Pandemic H1N1 Origins • Derived from several viruses circulating in swine • New strain is probably a result of the reassortment of two swine influenza viruses, one from North America and one from Europe • North American virus already carried an avian and a human gene. • The new H1N1 virus has genes from swine, avian, and human influenzas Reassortments resulting in the current gene complement in the pandemic 2009 H1N1 virus. Figure from Garten, RJ, et al. 2009. Antigenic and Genetic Characteristics of Swine-Origin 2009 A(H1N1) Influenza Viruses Circulating in Humans. Science 325, 197-201. Flu vaccines: What’s in them? • Each seasonal influenza vaccine contains 2 influenza A viruses and 1 influenza B virus. • Data is gathered from 94 countries and analyzed by 4 WHO centers (USA, UK, Australia, & Japan). WHO makes recommendations in February for vaccines for Northern Hemisphere. • Strains are selected based on forecasts about which are most likely to cause disease in the coming flu season. Vaccine production • Manufacturers grow the 3 strains in eggs or in chicken kidney cells (3 strains trivalent vaccine) • It takes 6 months to grow sufficient quantities of virus for vaccine preparation • Novel H1N1 strain (H1N1/09) developed too late to be included in the annual influenza vaccine • H1N1 vaccine was prepared in the same way as the seasonal influenza vaccinejust separately! What are the reagents? Purified antigen: Chicken gamma globulin Primary antibody: Polyclonal anti-chicken antibody made in rabbits Enzyme-linked secondary antibody: Polyclonal anti-rabbit antibody (made in goats) linked to horseradish peroxidase (HRP) Enzyme substrate: TMB (3,3’,5,5’-tetramethylbenzidine) - a colorless solution that turns blue when oxidized by HRP Ways The ELISA Kit Can Be Used Protocol Type of ELISA I Tracking outbreaks of disease Detecting antigens II III Detecting antibodies in serum Real-World Application HIV, Bird Flu and West Nile viruses, common cold, cholera, smallpox, anthrax, and STDs Pregnancy, drug, GMO and allergen tests Air food and water testing Influenza, HIV, smallpox, West Nile and Flu viruses HIV, Lyme disease, trichinosis, West Nile virus, and Flu virus Objectives Epidemiology, disease spread, public health Uses for antibodies in research, medicine, and consumer goods Detecting exposure to disease causing agents Webinars • Enzyme Kinetics — A Biofuels Case Study • Real-Time PCR — What You Need To Know and Why You Should Teach It! • Proteins — Where DNA Takes on Form and Function • From plants to sequence: a six week college biology lab course • From singleplex to multiplex: making the most out of your realtime experiments explorer.bio-rad.comSupportWebinars