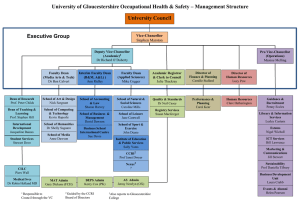
E. coli - National Centre for Biotechnology Education
advertisement

The Lambda protocol National Centre for Biotechnology Education Copyright © Dean Madden, 2012 www.ncbe.reading.ac.uk Lambda DNA Lambda phage E. coli DNA E. coli Lambda DNA enters cell and forms a ring Lysogenic cycle Lytic cycle Activation Lambda DNA integrates within the host E. coli chromosome UV light Lambda DNA replicated Lysis Copyright © Dean Madden, 2012 E. coli bursts open, releasing new lambda phages Head with DNA Tail Non-essential region Copyright © Dean Madden, 2012 Lambda DNA 48 502 base pairs BamHI Copyright © Dean Madden, 2012 BamHI Copyright © Dean Madden, 2012 EcoRI Copyright © Dean Madden, 2012 HindIII Copyright © Dean Madden, 2012 HindIII 2027 23103 564 2322 9416 6557 125 4361 BamHI 5505 16841 5626 6527 7233 6770 EcoRI 21226 4878 5643 7421 5804 3530 Copyright © Dean Madden, 2012 Copyright © Dean Madden, 2012 Summary of procedure Copyright © Dean Madden, 2012 Microsyringe HOLD HERE Do not touch the point! Graduated tip 10 µL Copyright © Dean Madden, 2012 2 µL Mass A microgram is one millionth of a gram 1 000 micrograms (µg) = 1 milligram (mg) 1 000 milligrams = 1 gram (g) Volume A microlitre is one millionth of a litre 1 000 microlitres (µL) = 1 millilitre (mL) 1 000 millilitres = 1 litre (L) Copyright © Dean Madden, 2012 Cap the tube firmly Add 100 µL of distilled water Copyright © Dean Madden, 2012 30 µg of dried lambda DNA Flick the tube repeatedly to dissolve the DNA Mix the DNA solution thoroughly before dispensing it Lambda DNA solution 20 µL 20 µL 20 µL 20 µL Use a new tip to add the DNA solution to each tube, to prevent contamination BamHI HindIII Empty Copyright © Dean Madden, 2012 EcoRI Close the tubes and push them firmly into the foam floater Copyright © Dean Madden, 2012 Incubate for 30–45 minutes at 37°C Carbon fibre tissue 42 mm Cut two electrodes Copyright © Dean Madden, 2012 22 mm Frosted panel on this side Molten agarose 55–60 °C Copyright © Dean Madden, 2012 Copyright © Dean Madden, 2012 Pour buffer over the gel before removing the comb Mix loading dye into each DNA sample 2 µL 2 µL 2 µL 2 µL Copyright © Dean Madden, 2012 TBE buffer solution 2–3 mm depth of buffer over the gel Label the end of the tank to show the contents of each well Black card under the tank reveals the wells for loading Copyright © Dean Madden, 2012 Electrodes Copyright © Dean Madden, 2012 Place a comb over the tank to reduce evaporation Direction of DNA movement Copyright © Dean Madden, 2012 Azure A stain Leave the stain on for 4 minutes only Copyright © Dean Madden, 2012 Positively-charged Azure A binds to the negatively-charged phosphate groups of the DNA DNA Copyright © Dean Madden, 2012 Wells Millimetre graph paper beneath the gel is a convenient way to measure distances Loading dye Area with DNA bands Copyright © Dean Madden, 2012 Specimen results EcoRI HindIII BamHI Copyright © Dean Madden, 2012 Uncut