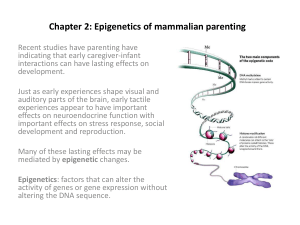
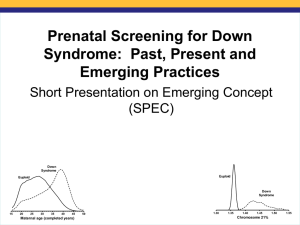
Montefiore Medical Center 2011: Prenatal Screening Using Free
advertisement

Prenatal Screening Using Free DNA in Maternal Blood Jacob Canick, PhD Alpert Medical School of Brown University Women & Infants Hospital Providence, RI, USA Department of Pathology Montefiore Medical Center Bronx, NY March 17, 2011 Declaration of Interests Pertinent to this Discussion Funding from SEQUENOM, Inc., San Diego, CA, to conduct a clinical study on tests for trisomy 21 in pregnancy using fetal nucleic acids in maternal plasma. Current Screening Uses Prenatal Markers of the Down Syndrome Phenotype • The best test performance is currently: 95% DR @ 5% FPR or Full/Sequential Integrated Test 90% DR @ 2% FPR 85% DR @ 5% FPR 1st Trim. Combined or Serum Integrated Test 80% DR @ 5% FPR 2nd Trim. Quad Test • All screen positive women should be counseled on risks and benefits of invasive procedures for karyotype analysis. Current Screening for fetal Down Syndrome using Phenotypic or “Surrogate” Markers Fetal ultrasound: Maternal Serum: PAPP-A low Nuchal Translucency increased AFP low Nasal Bone absent/small uE3 low Nuchal fold thickness larger Femur/Humerus shorter b-hCG elevated inhibin A elevated Echogenic cardiac focus present Ductus venosus doppler reversed a-wave Nuchal Translucency Nasal Bone Ductus venosus doppler www.fetalmedicine.com/fmf Future Prenatal Testing: Prenatal Screening and Diagnosis of Fetal Trisomies New direction: direct identification of the disorder (markers of genotype rather than markers of phenotype). Targeted to the specific numerical chromosomal disorder: Trisomy 21 rather than Down syndrome phenotype Trisomy 18 rather than Edwards syndrome phenotype Trisomy 13 rather than Patau syndrome phenotype Measure specific free fetal nucleic acids (DNA or RNA) in the maternal circulation. Background: Fetal Nucleic Acids in Maternal Plasma • First report of free fetal DNA in maternal circulation. (Lo YMD et al. Lancet 1997;350:485-7) • Fetal DNA clears rapidly from maternal circulation after the baby is delivered. (Lo YMD et al. Am J Hum Genet 1999;64:218-24) • First report of free fetal RNA in maternal circulation. (Poon LLM et al. Clin Chem 2000;46:1832-4) • Prenatal diagnosis of fetal RHD status by molecular analysis of maternal plasma. (Lo YMD et al. N Engl J Med 1998;339:1734-8) Cell-free DNA in the Maternal Circulation Placenta Maternal plasma Maternal blood cells • Both cell-free fetal and cell-free maternal DNA circulate in maternal plasma. • Cell-free fetal and maternal DNA circulate in maternal plasma as relatively short fragments (150-200 base pairs) and represent the entire genome. • Fetal DNA comes primarily from the placenta. • Maternal DNA comes primarily from maternal blood cells. • Fetal DNA is 5-25% of the total cell-free DNA (~10% on average). Potential clinical applications of analysing fetal nucleic acids in maternal plasma. Lo and Chiu, Nature Reviews Genetics 2007 Massively Parallel Sequencing (MPS): Identifying Down syndrome using circulating cell free DNA in maternal plasma First publications on MPS for trisomy 21 detection PNAS 2008;105:15255 PNAS 2008;105:20458 The Concept 10% of free DNA in maternal plasma is fetal Relative amount of chromosome 21 Normal Mother Normal Fetus 18 copies + 2 copies 20 copies Relative amount of chromosome 21 Normal Mother Down syndrome Fetus 18 copies + 3 copies 21 copies Need to distinguish 21 copies from 20 copies, a 5% difference. (assumes 10% of ccfDNA is fetal) But, fetal and maternal DNA are not distinguishable by MPS Relative amount of chromosome 21 18 copies + 2 copies 20 copies Relative amount of chromosome 21 18 copies + 3 copies 21 copies Need to distinguish 21 copies from 20 copies, a 5% difference. (assumes 10% of ccfDNA is fetal) Schematic illustration of the procedural framework for using massively parallel genomic sequencing for the noninvasive prenatal detection of fetal chromosomal aneuploidy. Chiu R W K et al. PNAS 2008;105:20458 Schematic illustration (con’t) %chr21 = 1 / 51 = 2% Chiu R W K et al. PNAS 2008;105:20458 Schematic illustration (con’t) • For each chromosome, determine its average % of unique sequences, compared to the total number of sequences in the normal human genome. • Do this by getting data from many ‘normal’ samples. • This will produce a normal distribution (mean ± SD) for each chromosome. • For example: % unique sequences in chromosome 21 in six different euploid genomes 2.01 2.00 1.98 2.02 2.03 1.99 2.01 ± 0.02 (mean ± standard deviation) Schematic illustration (con’t) mean .… -6 -5 -4 4 5 6…. Z score (± SD) schematic from www.sci.sdsu.edu Schematic illustration (con’t) To test an individual: Determine the % of chromosome 21 unique sequences for that person and compare that % to the mean, in terms of ± SD (Z Score). Z score calculation % unique sequences in chromosome 21 in euploid 2.01 2.00 1.98 2.02 2.03 1.99 -----2.01 ± 0.02 in test sample Z = (2.11 – 2.01) 0.02 2.11 Z= 0.10 0.02 Z= 5 Chiu R W K et al. PNAS 2008;105:20458 Schematic illustration (con’t) euploids trisomy 21 cases Chiu R W K et al. PNAS 2008;105:20458 How is this implemented? Four steps in the MPS process 1. Library Preparation • Purify free DNA from maternal plasma (already fragmented) • Add special adapters to both ends • Dilute to get proper concentration range 2. Cluster Generation • Run samples through Illumina flow cell (8 lanes per cell) to capture fragments • Solid-phase amplification of fragments to generate clusters 1 2 3 4 Four steps in the MPS process 3. Sequencing by Synthesis • • • • Illumina High Seq 200, a pumping and imaging system Sequence the first 36 bases >10 million clusters sequenced per flow cell lane >1 terabyte of data per flow cell 4. Data Analysis • Alignment (chromosome matching) using human genome database • One matching error per 36 bases allowed • Interpretation of results: % of matches on chromosome 21 Z score for each sample Published results so far… Proportion of unique sequences per chromosome, from three plasma samples and genome database Unique matches (%) Bars (Left to Right) Expected genomic % Normal female fetus Dup NFF, protocol 2 Normal male fetus Dup NMF, protocol 2 Mix of 2 norm males Dup Mix, protocol 2 Chromosome Number Chiu R W K et al. PNAS 2008;105:20458 Proportion of unique sequences per chromosome, from three plasma samples and genome database Bars (Left to Right) Expected genomic % Normal female fetus Dup NFF, protocol 2 Normal male fetus Dup NMF, protocol 2 Mix of 2 norm males Dup Mix, protocol 2 Chiu R W K et al. PNAS 2008;105:20458 Black Blue Orange Green Red genomic representation normal male normal female T21 male T21 female Z-score % of all unique reads Percent unique reads and corresponding z-score for chromosome 21, on 28 maternal plasma samples Normal range Chiu R W K et al. PNAS 2008;105:20458 Z scores for each chromosome New publications on MPS for trisomy 21 detection Chiu et al. 8-plex 86 cases 571 controls DR: 79% FPR: 1% 2-plex 86 cases 146 controls DR: 100% FPR: 2% Ehrich et al. monoplex 39 cases 410 controls DR: 100% FPR: 0.3% Independent Clinical Trial Nearing Completion BROWN Women & Infants’ • Enrolled pregnant women, from 27 Recruitment Sites worldwide, were at high risk based on prenatal screening, abnormal fetal ultrasound, age >38 years. • All enrollees had maternal plasma samples taken prior to CVS or amniocentesis; sample processing within 6 hours. • More than 4500 women enrolled, with more than 200 cases of fetal trisomy 21 (half 1st trim, half 2nd trim.) • Other aneuploidies are also studied. • Testing of coded samples by Massively Parallel Sequencing of free DNA in the maternal plasma at SCMM. • Funded by Sequenom Inc. Free DNA-based Testing for Trisomy 21: Further Issues • Cost hundreds, thousands of $$$$? getting less expensive very quickly • Turnaround time 3 days, 7 days, longer? • Availability limited lab sites intellectual property issues • Amnio/CVS still necessary? Is it diagnostic, or just a very good screening test? Conclusions • Current methods of prenatal screening reach a performance of 90% DR at a 5% FPR. • Measurement of free DNA in the maternal circulation holds the possibility for considerably better screening performance, perhaps even non-invasive diagnosis. • Currently, massive genomic sequencing appears to hold the most promise. • Other chromosomal aneuploidies should be able to be identified by this approach. • Other genetic defects, including single gene disorders, may also be identified by this approach. Fetal DNA Study Collaborators Women & Infants Hospital/Brown University: Glenn Palomaki, PhD Ed Kloza, MS Geralyn Lambert-Messerlian, PhD Regina Traficante, PhD UCLA School of Medicine: Stan Nelson, MD Wayne Grody, MD, PhD Sequenom Center for Molecular Medicine: Mathias Ehrich, MD Dirk van den Boom, PhD Allan Bombard, MD and investigators at 27 sites in NA, SA, Europe, Australia