Characterization of the protein recognized by the monoclonal
advertisement

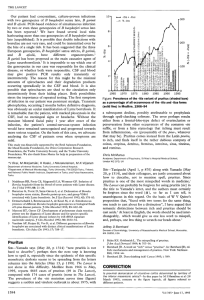
Characterization of the protein recognized by the monoclonal antibody D6 specific for Borrelia garinii isolates 1O. Péter*, 1 J-C. Wyss, 1A.G.Bretz, 1L. Toutoungi, 2A. Scherl, 1X. Zhang, 2J.-C. Sanchez, 3R. Sahli 1 Institut Central des Hôpitaux Valaisans, Microbiologie (ICHV), Service of Infectious Diseases, 1950 Sion, Switzerland 2 Biomedical Proteomics Research Group, Department of Bioinformatics and Structural Biology, Geneva University, 1200 Geneva 3 Centre Hospitalier Universitaire Vaudois (CHUV), Institute of Microbiologie, 1011 Lausanne Background : Methods : In Europe, Borrelia burgdorferi sensu lato isolates belong to 4 major species: B. burgdorferi sensu stricto, B. afzelii, B. garinii and B. valaisiana. The objective of this study was to characterize low molecular weight proteins of B. burgdorferi sensu lato. Our main focus was a protein around 12 kDa, that is reactive with D6, a monoclonal antibody specific for B. garinii isolates. Proteins of a B. garinii isolate (VS 102) were prepared as described schematically below. The Bb477, Bb061, Bb390 open reading frames of 28 isolates (5 B. burgdorferi sensu stricto, 5 B. afzelii, 13 B. garinii and 5 B. valaisiana) was analysed by PCR and DNA sequencing using the BigDye chemistry. Sequence alignments were done with clustalw included in the Vector NTI advance package (Invitrogen). Genes were cloned and expressed in E. coli PQR9. Putative epitope sequence was synthezised and mab D6 was saturated and to observe absence of reactivity. Amplification by PCR of 13-28 Borrelia isolates Tandem mass spectrometry analysis Mascot Search Search in Genbank Design of primers for PCR Sequencing of amplicons with ABI 310 instrument rpsJ B31 B VS123 A ACA1 VS215 A26s Expression of rpIL and TrxA, in E. coli PQR9 VS461 935T 387 G NT29 VS102 Recombinants rpIL and TrxA, dilutions (1)1/2, (2)1/20, (3)1/200, (4)1/2000 Immuno-absorption of mab D6 with synthetic peptide (TrxA 4-20) UK V Results : Partial sequences of 3 proteins of Borrelia burgdorferi were obtained from the tandem mass spectrometry analysis: Bb477 (30S ribosomal protein S10, rpsJ), BB061 (thioredoxine A, TrxA), Bb390 (50s ribosomal protein L7/L12, rpIL). All 3 complete genes were amplified by PCR from 13 to 28 isolates. DNA sequences were translated and aligned. VS116 AG1 C+ C+ TrxA B B31 VS123 VS215 A VS461 ACA1 A26s 935T C+ 1 2 3 4 1 2 ……..rpIL…………….. TrxA 3 4 387 20047 A19s A77c G FAR01 G25 M63 VS BM VS BP HP3 NT19 VS102 V UK VS116 AG1 rpIL B31 B VS123 VS215 Geho IP1 VS461 ACA1 A A26s A38s Bo23 935T 387 20047 A19s G A77c FAR01 G25 M63 VS BM VS BP HP3 NT29 VS102 VS116 V UK AG1 F10.08.94 FRANK Conclusion 1: Conclusion 2: Conclusion 3: After purification of low molecular mass proteins of B. garinii, partial sequences of 3 proteins of Borrelia burgdorferi were obtained from the mass spectrometry analysis: Bb390 (50s ribosomal protein L7/L12, rpIL), Bb477 (30S ribosomal protein S10, rpsJ), BB061 (thioredoxine A, TrxA). All 3 complete genes were amplified by PCR from 13 to 28 Borrelia isolates belonging to B. burgdorferi, B. afzelii, B. garinii and B. valaisiana. TrxA and rpIL were expressed in E. coli PQR9 and mab D6 showed weak reactivity to recombinant TrxA protein and no reactivity to recombinant rpIL. Based on sequence alignements, epitope was suspected to be in position 7-12 of TrxA A synthetic peptide was ordered and was used for immuno-absorption. It confirmed that epitope of mab D6 corresponds to KEDFVA sequence of Thioredoxyne A protein (amino acids 7-12).