A Potential Mechanism of Virus Persistence within Bivalve Shellfish
advertisement

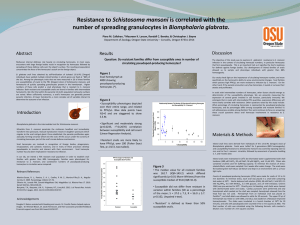
Persistence of Enteric Viruses within Oysters (Crassostrea virginica) David Kingley1 and Keleigh Provost2 1USDA ARS FSIT Dover Laboratory 2College of Agric. and Related Sciences, Delaware State Univ. USDA Microbial Safety of Aquaculture Products Center of Excellence Located within Department of Agriculture and Related Sciences, Delaware State University, Dover DE DHK’s USDA Laboratory Research Enhance shellfish safety by developing rapid methods for detection of viruses Understanding of how and why viruses persist within bivalve tissues (hemocytes) Identify and evaluate potential intervention technologies to eliminate or inactivate pathogenic viruses from shellfish Virus Transmission Fecal-oral route: Person-to-Person, Contaminated Water, Food handlers, Shellfish Do Not Replicate in Food Items Cold, Dark, and Wet concept Small infectious dose (10-100 virions for HAV and Norwalk?) Sources of Enteric Viruses Septic systems Raw/Treated Sewage Overboard waste disposal Floods Variable HPP results HAV 3-log10 reduction at 400 MPa in oysters MNV 400 MPA in oysters reduces 3-log10 HuNoV 600 MPa required inactivate 10,000 GEC units (Human volunteers) in oysters (400 MPa unsuccessful) High Pressure Processing and Viruses: Take Home Messages HPP can kill HAV and NV – requires pressures above commercial range Pressure level is predominate; time secondary Temperature is important: For NV surrogates-cold enhances; for HAV cold is protective Food Matrix effects are important Some food-borne viruses may be resistant Exceptions are the rule (pH and temp) Bioconcentration vs. Persistence Bivalve shellfish bioconcentrate water-borne pathogens to high levels (1000x) Fecal bacteria are readily purged; fecal viruses are not (why not?) Some viruses persist in shellfish better than others (?) IntroductionWhy do enteric viruses persist in shellfish? Sequestration within hemocytes Relationship between virus testing for whole oysters and hemocytes Demonstrate virus persistence within hemocytes-relationship with acid tolerance HAV persists in shellfish Hemocytes are involved in: http://www.mdsg.umd.edu/issues/chesapeake /oysters/education/oysblood.htm Wound repair Nutrient transport Innate immune defense Digestion of food particles Hemocytes are phagocytic Phagolysosomes are acidic Waste ejected Sequestration within hemocytes MW f-HL H-pell HL-unf + (-) Virus associated with hemocytes not hemolymph Separation of hemocytes from hemolymph. Oysters were contaminated with 4.3 x 107 PFU of HAV for O/N and then hemocytes and hemolymph were extracted. Hemocytes were separated from hemolymph by centrifugation and the supernatant was tested or filtered. Testing by extraction of the viral RNA and RT-PCR. Persistence within hemocytes (H) and whole oyster (W) MW H5 W5 H9 W9 H15 W15 (+) (-) Days 5-15. This is a representative gel for three trials. Whole shucked oysters and hemocyte samples were obtained after exposure to poliovirus, 1 ml of 1.12 x 108 pfu/ml, and depuration for varying periods. Persistence within hemocytes (H) and whole oyster (W) MW H29 W29 (+) (+) (-) Persistence of HAV in extracted tissues and hemocytes. Whole shucked oyster meat or hemocytes were extracted after exposure to HAV (4.28 x 107 pfu/ml) and depuration for varying lengths of time. Lane 1 is a 100 Bp DNA ladder. Lane 3 is a hemocyte sample from 29 days post-exposure, lane 4 is a whole oyster sample from 29 days postexposure. Lane 5 is a positive control, with 1 μl of denatured virus, 8 μl of water and 1 μl of RNAse inhibitor. Lane 6 is also a positive RT-PCR virus control with 1 μl of column run HAV RNA, and 9 μl of water. Lane 6 is a negative RT-PCR control with 10 μl of water. Exposure of all 4 viruses at once Same 3 x 106 RT-PCR units of virus o/n; two separate trials; individual oysters FCV detected at day 0 only PV detected at day 1 only MNV detected at day 3 and day 12 HAV beyond 21 days Most persistent HAV >MNV >PV >FCV least persistent Caveat: GPTT extraction probably not equivalent for all viruses pH Sensitivity *30 min treatment 2 PV HAV HAV 0 FCV Log Reduction MNV FCV -2 FCV MNV PV -4 FCV -6 FCV Polio HAV MNV -8 PV pH1 pH2 pH3 pH4 pH5 pH Treatments Most Acid Resistant HAV, >MNV, >PV, >FCV Least Acid Resistant Persistence within hemocytes Viable HAV and PV was isolated from hemocytes Hemocytes (+) whole oysters (+) Relationship between acid tolerance and persistence Slow release to hemocytes (?) Transfer experiments (no adaptive immune system) Testing whole oysters after transfer of HAV-contaminated hemocytes MW C oy 1W 2W 3W H (+) (-) Whole oysters test positive for HAV two weeks after hemocyte transfer HAV persistence after transfer of contaminated hemocytes to naïve oysters. (several oysters 4 x107 pfu o/n pooled then transfer ) Lane 1 is a 100 bp DNA ladder. Lane 3 is a control of the naïve oyster, before the transfer. Lane 4 is a whole oyster sample 1 week post-transfer. Lane 5 is whole oyster sample 2 weeks post-transfer. Lane 6 is a whole oyster sample 3 weeks post-transfer. Lane 7 is a subsample of the contaminated hemocytes before injection. Lane 8 is a positive RTPCR control,. Lane 9 is a negative RT-PCR Conclusion Relationship between low pH tolerance and persistence within shellfish meats Virus is associated with hemocytes HAV can remain within hemocytes for extended periods Hemocytes: Research Directions and Implications More detailed research-visualize within hemocytes (phagolysosomes) …track virus movement thru the oyster Hemocytes appear to be a good detection target Design new intervention strategies??