Molecular Quality Improvement Program

CDC Services
Suzanne Cordovado, Ph.D.
Team Lead, Molecular Quality Improvement Program
Newborn Screening and Molecular Biology Branch,
Division of Laboratory Sciences
NCEH, CDC
Tuesday, 28th June 2011
National Center for Environmental Health
Centers for Disease Control and Prevention
Newborn Screening and Molecular
Biology Branch Organization
Newborn Screening and
Molecular Biology Branch
NSMBB
(6 teams)
Newborn
Screening Quality
Assurance
Program
(NSQAP)
Newborn
Screening
Translation
Research Initiative
(NSTRI)
Biochemical Mass
Spectrometry
Laboratory
(BMSL)
Molecular Quality
Improvement
Program
(MQIP)
The Newborn Screening
Quality Assurance Program
The only comprehensive quality assurance program using the dried-blood spots
Laboratory Services Provided by NSQAP
1. Filter paper evaluation
2. DBS reference and quality control materials
3. Proficiency Testing
4. Internet reporting site for laboratories
5. Follow-up of False negative results
6. Training, consultation, network resources
Translation Research Initiative
Mission:
Assure that the translation of research methods into routine laboratory tests for newborn screening leads to sustainable high-quality testing and healthier babies worldwide
Develop new screening methods for specific diseases
Integrate State Public Health Laboratories into the translation process through collaborative field studies
Expand global reach of newborn screening
Adapt innovative technologies for screening and quality assurance
Ongoing collaboration between the CDC Foundation and NSMBB
Biochemical Mass Spectrometry Laboratory
Mission Statement:
Work with public health partners to develop new mass spectrometrybased assays to detect and monitor metabolic disorders, and enhance newborn screening laboratory performance through innovative approaches to biochemical marker detection.
Selected Priorities:
Develop new methods for the analysis of driedblood for metabolic screening and diagnosis of selected inborn errors of metabolism
Pilot program for MS/MS analyte ratios analysis for metabolic disorders to improve specificity of existing MS/MS-based newborn screening assays
Molecular Quality Improvement Program
Mission:
Work with public health laboratories to detect newborn disorders with molecular methods, and provide a public health forum to exchange molecular best practices, quality improvements and educational resources to enhance laboratory performance.
Molecular screening brings new and different technologies into the NBS laboratory creating a need for newborn screening laboratory resources
Second tier and primary molecular methods are now being used by a number of newborn screening laboratories
NBS Molecular Testing Status: 2010
36 state labs (denoted in green) offer a molecular test
84% of babies born/year
Molecular Quality Improvement Activities
Establishment of the NBS Molecular Network
Implementation of NBS Molecular Assessment Program
(MAP)
QA research to identify and develop quality molecular methods for the DBS matrix
Molecular characterization of quality assurance (QA) materials ( e.g
. cystic fibrosis and hemoglobinopathies)
Translational research to address NBS community identified needs and QA protocols
Establishment of the NBS Molecular Network
Public health partners working synergistically to enhance newborn screening with molecular tests
2010 NBS and Genetic Testing Symposium
Facilitate a collaborative environment for knowledge and technology transfer between
NBS labs
Assist labs in improving sensitivity and/or specificity of detection using primary or second-tier molecular tests
Collaborate with NBS labs to anticipate future molecular assays and needs
NBS Molecular Network Goals
Steering committee is comprised of
NSMBB staff, Public Health partners and
APHL
1 st meeting – February 23-24, 2011, Atlanta, GA
Goal 1: Prioritize disorders to implement molecular tests to enhance screening
Goal 2: Identify collaborative projects to fill gaps in molecular NBS
Goal 3: Plan strategies to enhance communication, education and dissemination of best molecular lab practices
Quality Assurance Research:
Evaluation of DNA Extracted from DBS in NBS Assays
Determine DBS DNA extraction efficiency of commercially available extraction methods
Test downstream assay performance in NBS laboratories
Cystic fibrosis – InPlex CF (Hologic) and xTAG CF 39 (Luminex)
Galactosemia – in-house GALT assay
Hemoglobinopathies – in-house HBB assay
SCID – in-house TREC assay
State public health partners: California, Massachusetts, New
York, Texas, Washington, and Wisconsin
*
*MQIP/NSMBB/CDC
Quality Assurance Research:
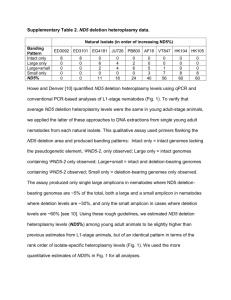
CFTR Inplex Optimization for DNA from DBS
Collaboration with Delaware NBS Laboratory
Determine minimum volume of input DNA from DBS for InPlex CF assay
DBS DNA is not routinely quantitated
Recommended input volume is 5 µl
Results indicate robust genotypes with input volumes > 2 µl
120.00%
Samples tested: newborn DBS and NSQAP
100.00%
CFTR PT DBS
Analysis suggests probe sets 3905insT,
120.00%
Low Probe Set Signals in Failed Samples by Sample Type and Volume
Low Probe Set Signals in Failed Samples
100.00%
80.00%
60.00%
20.00%
CDC failures @ 1 ul (n=9)
CDC failures @ 0.5 ul (n=19)
NBS failures @ 1 ul (n=5)
NBS failures @ 0.5 ul (n=20)
0.00% when input DNA volume < 2 µl
60.00%
40.00%
Mutation Probe Set for which more than one sample had a low signal
NSQAP failures @ 1 ul (n=9)
NSQAP failures @ 0.5 ul (n=19)
NBS failures @ 1 ul (n=5)
NBS failures @ 0.5 ul (n=20)
20.00%
0.00%
Mutation Probe Set for which more than one sample had a low signal
Future MQIP Projects
Web-based NBS molecular lab resource center
Guidelines/troubleshooting/education for molecular NBS assays
Discussion forum
Implementation of the Molecular Assessment
Program
Provide quality management guidance and support for molecular testing in NBS laboratories
Partnership between MQIP, NBS laboratories and APHL
Research and Development to expand molecular testing
Population mutation panels
Creation of QA materials and services
Future MQIP Projects cont.
Characterization of hemoglobinopathies in PT samples – Ghanaian Collaboration
Confirm abnormal hemoglobin gene variants
Expanded CF Repository
Sustain and expand CF PT repository to include currently unavailable mutations
Create DBS QC materials for assay development and validation
Molecular Quality Improvement Program
Team Members
Suzanne Cordovado
Christopher Greene
Laura Hancock
Miyono Hendrix
Team Lead
MAP Coordinator
Web & Research Specialist
Research Specialist
Stanimila (Mimi) Nikolova Research Specialist
SCordovado@cdc.gov
CGreene@cdc.gov
LHancock@cdc.gov
MHendrix@cdc.gov
SNikolova@cdc.gov
Sean Mochal
Daniel Turner
ORISE Fellow
ORISE Fellow
SMochal@cdc.gov
DTurner@cdc.gov
For more information please contact Centers for Disease Control and
Prevention
1600 Clifton Road NE, Atlanta, GA 30333
Telephone, 1-800-CDC-INFO (232-4636)/TTY: 1-888-232-6348
E-mail: cdcinfo@cdc.gov Web: www.cdc.gov
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
National Center for Environmental Health
Centers for Disease Control and Prevention