O-Iodoxybenzoic acid and related iodoxyarenes
advertisement
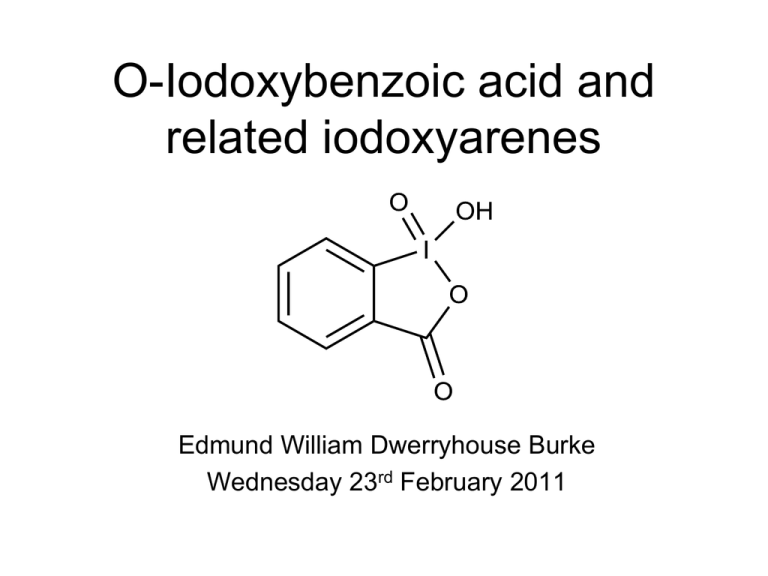
O-Iodoxybenzoic acid and related iodoxyarenes Edmund William Dwerryhouse Burke Wednesday 23rd February 2011 History • Discovered in at the end of the 19th century • Biologically tested and used as a medicine • Not really used until the end of the 20th Structure and bonding • O-iodoxybenzoic acid (IBX), exists in a single tautomeric form, IUPAC name; 1-hydroxy-1-oxo-1H-1l5-benzo[d][1,2]iodoxol-3-one • Planar except for O4, leading to chirality • Can be crystallize as a conglomerate • Octahedral geometry around iodine due to intermolecular I-O interactions S. F. Kirsch and A. Duschek, Angew. Chem. Int. Ed. 2011, 50, 1524 – 1552 More bonding • Hypervalent bonding – Hypervalent atom may have colinear single bonds – Two types of single bond from the same atom – Doubly occupied p-orbitals – Oxo ligand is associate via a dative bond J. I. Musher, Angew. Chem. Internat. Edit. Vol. 8 (1969), 54-68 S. F. Kirsch and A. Duschek, Angew. Chem. Int. Ed. 2011, 50, 1524 – 1552 Preparation • Numerous methods have been reported, either via oxidation of 2-iodosobenzoic acid or 2iodobenzoic acid. Method of choice is now: Marco Frigerio, Marco Santagostino, and Simona Sputore, J. Org. Chem. 1999, 64, 4537-4538 Advantages of IBX and its derivatives • • • • Environmentally safe Stable in presence of water and oxygen Can be regenerated Compatible with a wide range of common protecting groups • Does not over oxidise aldehydes to acids • Does not oxidise tertiary amines or heterocycles Disadvantages of IBX and its derivatives • Lack of solubility in most organic solvents • Explosive Overcome by •Polymer bound IBX •Use of SIBX, formulation of benzoic acid, 2-iodobenzoic acid, and IBX •Derivatives Marcel M¸lbaier and Athanassios Giannis, Angew. Chem. Int. Ed. 2001, 40, 4393-4394 Mechanism Hypervalent twist mechanism S. F. Kirsch and A. Duschek, Angew. Chem. Int. Ed. 2011, 50, 1524 – 1552 In situ generation of IBX C. Bulman Page, L. F. Appleby, B. R. Buckley, S. M. Allin, M. J. McKenzieb, Synlett 2007, No. 10, 1565–1568 Some Derivatives Jarugu Narasimha Moorthy, Nidhi Singhal and Kalyan Senapati, Tetrahedron Lett. 49 (2008) 80–84 S. F. Kirsch and A. Duschek, Angew. Chem. Int. Ed. 2011, 50, 1524 – 1552 Example use of a derivative 2 eq. DCM, rt. 1h 87 % Paul A. Grieco,' Jon L. Collins,* Eric D. Moher Thomas J. Fleck, and Raymond S. Gross, J. Am. Chem. SOC. 1993, 115, 6078-6093 Some examples using Me-IBX Jarugu Narasimha Moorthy, Nidhi Singhal and Kalyan Senapati, Tetrahedron Lett. 49 (2008) 80–84 Sulphides to sulphoxides Jarugu Narasimha Moorthy, Nidhi Singhal and Kalyan Senapati, Tetrahedron Lett. 49 (2008) 80–84 Single electron transfer mechanism for the oxidation of sulphides IBX and Diols • Also make hemiaminals, then lactams One pot reactions Fredy Leon, Daniel G. Rivera, and Ludger A. Wessjohann, J. Org. Chem., 73, 2008, 1762-1767 Dehydrogenation with IBX • Aromatization • Dehydrogenation of carbonyls K. C. Nicolaou, T. Montagnon, P. S. Baran, and Y.-L. Zhong, J. Am. Chem. Soc., 124, 2002, 2245-2258 Dearomatization of Phenols Derek Magdziak, Andy A. Rodriguez, Ryan W. Van De Water, and Thomas R. R. Pettus, Org. Lett., 4, 2002, 285-288 Evidence for the mechanism of Phenol dearomatization Summary • • • • Broad array of reactions Various reaction mechanisms Mild and selective oxidising agents Easy to use


![Cooper_Abstract_MOF_2014[1]](http://s3.studylib.net/store/data/006662442_1-973d7b3fb19ef7da02e38c87851e45c2-300x300.png)