R - Groupe Charette
advertisement
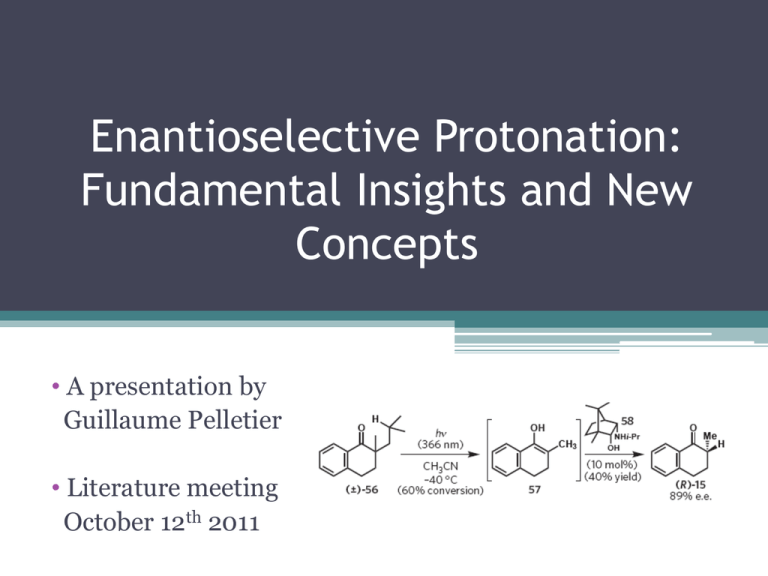
Enantioselective Protonation: Fundamental Insights and New Concepts • A presentation by Guillaume Pelletier • Literature meeting October 12th 2011 Enantioselective Protonnation : An Extremely Simple Transformation!(?) • Enolates are important as synthetic intermediates : regio and stereoselective generation with the desired counterion, increased knowledge of their structure and reactivity • Enantioselective protonnation via enol tautomerisation : require only catalytic amounts of chiral reagent. • Protonnation of a chiral enolate/ligand complex What is the Important Facts to Know Before Exploring «AP» of Enolates • Enantioselective protonation controlled reactions processes are necessarily kinetically • Match the pKa of the proton donnor and the product • Be concerned about the stereochemistry of the proton acceptor : the ability to generate a stereodefined proton acceptor is critical (or not) in order to have good enantioselectivity • Detailed mechanistic explanations are rare : mixture of many mechanisms Presentation Outline • Lucette Duhamel and J.-C. Plaquevent’s Asymmetric Protonation of Benzylidene Glycinates (1978) • Charles Fehr’s Synthesis of α- and γ-Damascone (1988) • Hisashi Yamamoto’s Catalytic Asymmetric Protonation of Silyl Enol Ether with LBA (1994) • Recent Contributions (Levacher, Genet, Fu, Stoltz…) (2005+) Fehr, C. Angew. Chem., Int. Ed. 1996, 35, 2566-2587. Eames, J.; Weerasooriya, N. Tetrahedron : Asymmetry 2001, 12, 1-24. Duhamel, L.; Duhamel, P.; Plaquevent, J.-C. Tetrahedron : Asymmetry 2004, 15, 3653-3691. Mohr, J. T.; Hong, A. Y.; Stoltz, B. M. Nature Chem. 2009, 1, 359-369. First « Synthetically Useful » Example of AP with Substituted Benzylidene Glycinates Duhamel, L.; Plaquevent, J. C. J. Am. Chem. Soc. 1978, 100, 7415-7416. Duhamel, L.; Plaquevent, J. C. Bull. Soc. Chim. Fr. 1982, II-75-83. Duhamel, L. et al. Tetrahedron 1988, 44, 5495-5506. Influence of the Chiral Acid Duhamel, L.; Plaquevent, J. C. J. Am. Chem. Soc. 1978, 100, 7415-7416. Duhamel, L.; Plaquevent, J. C. Bull. Soc. Chim. Fr. 1982, II-75-83. Duhamel, L. et al. Tetrahedron 1988, 44, 5495-5506. Influence of the Tartaric Acyl Substituents Entry R3 Yield (%) ee (%) [α]D25 1 Me 85 2.6 ‒2.2 (S) 2 i-Pr 85 12.1 ‒10.5 (S) 3 t-Bu 85 50 ‒41.9 (S) 4 1-adamantyl 79 53.2 -44.7 (S) 5 Ph 80 12.3 -10.3 (S) 6 CH2Ph 81 8.5 -6.95 (S) 7 (CH2)2Ph 83 6.5 +0.4 (R) Duhamel, L.; Plaquevent, J. C. J. Am. Chem. Soc. 1978, 100, 7415-7416. Duhamel, L.; Plaquevent, J. C. Bull. Soc. Chim. Fr. 1982, II-75-83. Duhamel, L. et al. Tetrahedron 1988, 44, 5495-5506. Influence of the Amino Acid Side-Chain Duhamel, L.; Plaquevent, J. C. J. Am. Chem. Soc. 1978, 100, 7415-7416. Duhamel, L.; Plaquevent, J. C. Bull. Soc. Chim. Fr. 1982, II-75-83. Duhamel, L. et al. Tetrahedron 1988, 44, 5495-5506. Influence of the Benzylidene Electronic Properties Entry R2 Yield (%) ee (%) 1 p-CN 75 12.3 2 p-Cl 75 31.3 3 H 85 50 4 p-CH3 82 55 5 o-OMe 70 36.6 6 p-OMe 70 57 7 p-NMe2 75 61 Duhamel, L.; Plaquevent, J. C. J. Am. Chem. Soc. 1978, 100, 7415-7416. Duhamel, L.; Plaquevent, J. C. Bull. Soc. Chim. Fr. 1982, II-75-83. Duhamel, L. et al. Tetrahedron 1988, 44, 5495-5506. Influence of the Base Additive Duhamel, L.; Plaquevent, J. C. J. Am. Chem. Soc. 1978, 100, 7415-7416. Duhamel, L.; Plaquevent, J. C. Bull. Soc. Chim. Fr. 1982, II-75-83. Duhamel, L. et al. Tetrahedron 1988, 44, 5495-5506. Results Interpretation Duhamel, L.; Plaquevent, J. C. J. Am. Chem. Soc. 1978, 100, 7415-7416. Duhamel, L.; Plaquevent, J. C. Bull. Soc. Chim. Fr. 1982, II-75-83. Duhamel, L. et al. Tetrahedron 1988, 44, 5495-5506. Enantioselective Protonation of Open-Chain Enolates Without Internal Chelating Atom • • • • Proton donnor should be only weakly acidic (pKa~15-20) Proton donnor should contain an electron-rich group with chelating ability The transferred proton should be located in the proximitiy of the stereogenic center Proton donnor should be readily accessible in both enantiomeric form and easily recoverable Fehr, C.; Galindo, J. J. Am. Chem. Soc. 1988, 110, 6909-6911. Fehr, C. Angew. Chem., Int. Ed. 1996, 35, 2566-2587. Enantioselective Protonation of Open-Chain Enolates Without Internal Chelating Atom • • • • Proton donnor should be only weakly acidic (pKa~15-20) Proton donnor should contain an electron-rich group with chelating ability The transferred proton should be located in the proximitiy of the stereogenic center Proton donnor should be readily accessible in both enantiomeric form and easily recoverable Fehr, C.; Galindo, J. J. Am. Chem. Soc. 1988, 110, 6909-6911. Fehr, C. Angew. Chem., Int. Ed. 1996, 35, 2566-2587. Enantioselective Protonation of Open-Chain Enolates Without Internal Chelating Atom • • • • Proton donnor should be only weakly acidic (pKa~15-20) Proton donnor should contain an electron-rich group with chelating ability The transferred proton should be located in the proximitiy of the stereogenic center Proton donnor should be readily accessible in both enantiomeric form and easily recoverable Fehr, C.; Galindo, J. J. Am. Chem. Soc. 1988, 110, 6909-6911. Fehr, C. Angew. Chem., Int. Ed. 1996, 35, 2566-2587. α-Damascone Synthesis – Ligand effect Enolate composition Enolate generation Yield (%) ee (%) 1 MgCl•MeOLi Grignard 60 58 2 MgCl Ketene 76 51 Entry Proton source α-Damascone Synthesis – Ligand effect Enolate composition Enolate generation Yield (%) ee (%) 1 MgCl•MeOLi Grignard 60 58 2 MgCl Ketene 76 51 3 MgCl Ketene N.D. 16 4 MgCl•MeOLi Ketene 75 70 5 MgCl•t-BuOLi Ketene 70 79 Entry Proton source α-Damascone Synthesis – Ligand effect Enolate composition Enolate generation Yield (%) ee (%) 1 MgCl•MeOLi Grignard 60 58 2 MgCl Ketene 76 51 3 MgCl Ketene N.D. 16 4 MgCl•MeOLi Ketene 75 70 5 MgCl•t-BuOLi Ketene 70 79 73 84 70 62 Entry Proton source 6 7 t-BuOH Ketene α-Damascone Synthesis – Enolate Stereoselectivity Effect Fehr, C.; Galindo, J. Angew. Chem., Int. Ed. 1994, 33, 1888-1890. Fehr, C. Angew. Chem., Int. Ed. 1996, 35, 2566-2587. α-Damascone Synthesis – Enolate Stereoselectivity Effect Fehr, C.; Galindo, J. Angew. Chem., Int. Ed. 1994, 33, 1888-1890. Fehr, C. Angew. Chem., Int. Ed. 1996, 35, 2566-2587. α-Damascone Synthesis – Enolate Stereoselectivity Effect Fehr, C.; Galindo, J. Angew. Chem., Int. Ed. 1994, 33, 1888-1890. Fehr, C. Angew. Chem., Int. Ed. 1996, 35, 2566-2587. γ-Damascone Synthesis – Effect of Alkoxide additives Fehr, C.; Galindo, J. J. Org. Chem. 1988, 53, 1828-1830. Fehr, C.; Galindo, J. Helv. Chim . Acta 1995, 78, 539-552. γ-Damascone Synthesis – Effect of Alkoxide additives Fehr, C.; Galindo, J. J. Org. Chem. 1988, 53, 1828-1830. Fehr, C.; Galindo, J. Helv. Chim . Acta 1995, 78, 539-552. γ-Damascone Synthesis – Effect of Alkoxide additives Fehr, C.; Galindo, J. J. Org. Chem. 1988, 53, 1828-1830. Fehr, C.; Galindo, J. Helv. Chim . Acta 1995, 78, 539-552. γ-Damascone Synthesis – Effect of Alkoxide additives Fehr, C.; Galindo, J. J. Org. Chem. 1988, 53, 1828-1830. Fehr, C.; Galindo, J. Helv. Chim . Acta 1995, 78, 539-552. γ-Damascone Synthesis – Effect of Alkoxide additives Equiv Entry H-A* Addition mode Solvent Li-A* (equiv) ee (%) at 25% Conv ee (%) at 50% Conv ee (%) at 100% Conv 1 1.2 normal THF/Et2O none 8 39 62 2 1.2 inverse THF/Et2O none 26 35 49 3 1.0 inverse THF/Et2O 1.0 - 65 68 4 1.0 inverse THF/Et2O 2.0 - 69 70 5 1.0 inverse THF 2.0 - 75 75 • The elucidation of the reaction mechanism is rendered more complex from the nonlinear relationship between reaction product and H-A* enantiomeric purity. Fehr, C.; Galindo, J. J. Org. Chem. 1988, 53, 1828-1830. Fehr, C.; Galindo, J. Helv. Chim . Acta 1995, 78, 539-552. γ-Damascone Synthesis – Effect of Alkoxide additives Fehr, C.; Galindo, J. Helv. Chim . Acta 1995, 78, 539-552. α and γ-Damascone Synthesis – Application to Thioester enolate Fehr, C.; Galindo, J. Helv. Chim . Acta 1995, 78, 539-552. Fehr, C.; Stempf, I.; Galindo, J. Angew. Chem., Int. Ed. 1993, 32, 1042-1044. α and γ-Damascone Synthesis – Application to Thioester enolate X Deprotonnation Protonnation B/A ee (%) Yield (%) OMe n-BuLi (1.5 equiv) -78 °C, 2.75 h (‒)-H-A* (2.0 equiv) -100 to 10°C,1.75h 22/78 36 (R) - 2 OMe LDA (3.0 equiv) -78 °C, 3 h (+)-H-A* (3.3 equiv) -100 to 10°C,2.25h 33/67 50 (S) - 3 OMe LDA (3.0 equiv) -78 °C, 3 h aq. HCl (excess), -78 °C 72/28 - - SPh n-BuLi (2.0 equiv) -78 °C, 3 h (‒)-H-A* (2.7 equiv) -100 to 10°C,1.75h 43/57 96 (R) 81 Fehr, C.; Galindo, J. Helv. Chim . Acta 1995, 78, 539-552 (‒)-H-A* (4.0 equiv) LDA (3.0 equiv) Fehr,5 C.; Stempf, I.; Galindo, J. Angew. Chem. Int. Ed. 1993, 32, 1042-1044. SPh 56/44 97 (R) 84 Entry 1 4 -78 °C, 2.75 h -100 to -10°C,1.5h α and γ-Damascone Synthesis – Application to Thioester enolate Fehr, C.; Galindo, J. Helv. Chim . Acta 1995, 78, 539-552. Fehr, C.; Stempf, I.; Galindo, J. Angew. Chem., Int. Ed. 1993, 32, 1042-1044. α and γ-Damascone Synthesis – Application to Thioester enolate Fehr, C.; Galindo, J. Helv. Chim . Acta 1995, 78, 539-552. Fehr, C.; Stempf, I.; Galindo, J. Angew. Chem., Int. Ed. 1993, 32, 1042-1044. α and γ-Damascone Synthesis – Application to Thioester enolate Fehr, C.; Galindo, J. Helv. Chim . Acta 1995, 78, 539-552. Fehr, C.; Stempf, I.; Galindo, J. Angew. Chem., Int. Ed. 1993, 32, 1042-1044. α-Damascone Synthesis – Catalytic Enantioselective Process • Slow and reversible generation of the transient enolate α-Damascone Synthesis – Catalytic Enantioselective Process • Slow and reversible generation of the transient enolate • Rapid and irreversible protonation of the enolate by H-A* α-Damascone Synthesis – Catalytic Enantioselective Process • Slow and reversible generation of the transient enolate • Rapid and irreversible protonation of the enolate by H-A* • The rate of regeneration of the catalyst and enolate can be ajusted with the external proton source (PhSH) • Proton exchange between A*- and PhSH must be rapid and complete and PhSLi must be more nucleophilic than Li-A* • Background reaction is suppressed by low [PhSH] α-Damascone Synthesis – Catalytic Enantioselective Process Temperature (°C) ArSH Addition time (h) ee (%) Yield (%) Entry ArSH Li-A* (mol %) 1 PhSH 100 -55 3 95 84 2 4-ClPhSh 100 -55 4 97 85 3 PhSH 5 -27 3 89 86 4 PhSH 2 -27 1 77 87 5 4-ClPhSH 5 -27 3 90 81 6 4-ClPhSH 2 -27 3 57 - Fehr, C.; Stempf, I.; Galindo, J. Angew. Chem., Int. Ed. 1993, 32, 1042-1044. Fehr, C.; Stempf, I.; Galindo, J. Angew. Chem., Int. Ed. 1993, 32, 1044-1046. Catalytic Enantioselective Protonation – General Scheme • With preformed enolates, [enolate] > [H-A*] • Formally, an external, achiral proton source Z-H selectively protonates A* and not the enolate • Protonation of A*- should be rapid with Z-H (unless there is a catalytic enantioselective tautomerisation mechanism) Fehr, C. Angew. Chem., Int. Ed. 1996, 35, 2566-2587. What About Preformed Enolates? (Autocalatylic) Fehr, C.; Galindo, J. Angew. Chem., Int. Ed. 1994, 33, 1888-1890. Fehr, C. Angew. Chem., Int. Ed. 1996, 35, 2566-2587. What About Preformed Enolates? (Autocalatylic) • This autocatalytic process is based on subtile kinetic differences in the proton transfer reactions between H-A*, A*-, the enolate and the noninducing proton donnor (Z-H). Fehr, C.; Galindo, J. Angew. Chem., Int. Ed. 1994, 33, 1888-1890. Fehr, C. Angew. Chem., Int. Ed. 1996, 35, 2566-2587. Catalytic Enantioselective Protonation – General Scheme Fehr, C.; Galindo, J. Angew. Chem., Int. Ed. 1994, 33, 1888-1890. Fehr, C. Angew. Chem., Int. Ed. 1996, 35, 2566-2587. Catalytic Enantioselective Protonation – General Scheme Fehr, C.; Galindo, J. Angew. Chem., Int. Ed. 1994, 33, 1888-1890. Fehr, C. Angew. Chem., Int. Ed. 1996, 35, 2566-2587. Catalytic Enantioselective Protonation – General Scheme Fehr, C.; Galindo, J. Angew. Chem., Int. Ed. 1994, 33, 1888-1890. Fehr, C. Angew. Chem., Int. Ed. 1996, 35, 2566-2587. Catalytic Enantioselective Protonation – General Scheme Entry H-A* (equiv) PhCH2Ac (equiv) ee (%) Yield (%) 1 1.1 - 96 90 2 0.2 0.85 94 94 3 0.1 0.95 85 99 4 0.2 0.8(TMSCl) 98 91 Catalytic Enantioselective Protonation – Protonnation of H-A*/enolate aggregate Fehr, C.; Galindo, J. Angew. Chem., Int. Ed. 1994, 33, 1888-1890. Catalytic Enantioselective Protonation of Cylic Lithium Enolates Yanagisawa, A.; Kuribayashi, T.; Kikuchi, T.; Yamamoto, H. Angew. Chem., Int. Ed. 1994, 33, 107-109. Yanagisawa, A.; Kikuchi, T.; Wanatabe, T.; Kuribayashi, T.; Yamamoto, H. Synlett 1995, 372-273. Yanagisawa, A.; Ishihara, K.; Yamamoto, H. Synlett 1997, 411-420. Catalytic Enantioselective Protonation of Cylic Lithium Enolates Kemp, D. S.; Petrakis, K. S. J. Org. Chem. 1981, 46, 5140-5149. Rebek, J., Jr.; Askew, B.; Killoran, M.; Nemeth, D.; Lin, F.-T. J. Am. Chem. Soc. 1987, 109, 2426-2433. Catalytic Enantioselective Protonation of Cylic Lithium Enolates *With a TMSCl quench at -78 °C! H-A* (equiv) ee (%) 1 1.0 87 2 0.10 83 3 0.05 72 4 0.10 90 5 0.01 81 6 0.10 88 7 0.01 80 Entry Achiral proton Yanagisawa, A.; Kikuchi, T.; Wanatabe, T.; Kuribayashi, T.; Yamamoto, H. Synlett 1995, 372-273. Yanagisawa, A.; Ishihara, K.; Yamamoto, H. Synlett 1997, 411-420. Catalytic Enantioselective Protonation of Cylic Lithium Enolates Yanagisawa, A.; Kikuchi, T.; Wanatabe, T.; Kuribayashi, T.; Yamamoto, H. Synlett 1995, 372-273. Yanagisawa, A.; Ishihara, K.; Yamamoto, H. Synlett 1997, 411-420. Enantioselective Protonation of Prochiral Silyl Enol Ethers and Ketene Silyl Acetals Ishihara, K.; Kaneeda, M.; Yamamoto, H. J. Am. Chem. Soc. 1994, 116, 11179-11180. Ishihara, K.; Nakamura, S.; Kaneeda, M.; Yamamoto, H. J. Am. Chem. Soc. 1996, 118, 12854-12855. Enantioselective Protonation of Prochiral Silyl Enol Ethers and Ketene Silyl Acetals • Silyl enol ether is a « stable metal enolate equivalent » which can be isolated • In general, it is difficult the control the enantioselectivity with protonation of silyl enol ether with chiral Brønsted acids • Two main reason for poor induction is bonding flexibility between H and A* and chiral pool of H-A* is limited to sulfonic and carboxylic acids Ishihara, K.; Kaneeda, M.; Yamamoto, H. J. Am. Chem. Soc. 1994, 116, 11179-11180. Ishihara, K.; Nakamura, S.; Kaneeda, M.; Yamamoto, H. J. Am. Chem. Soc. 1996, 118, 12854-12855. Enantioselective Protonation of Prochiral Silyl Enol Ethers Ishihara, K.; Kaneeda, M.; Yamamoto, H. J. Am. Chem. Soc. 1994, 116, 11179-11180. Ishihara, K.; Nakamura, S.; Kaneeda, M.; Yamamoto, H. J. Am. Chem. Soc. 1996, 118, 12854-12855. Enantioselective Protonation of Prochiral Silyl Enol Ethers Ishihara, K.; Kaneeda, M.; Yamamoto, H. J. Am. Chem. Soc. 1994, 116, 11179-11180. Ishihara, K.; Nakamura, S.; Kaneeda, M.; Yamamoto, H. J. Am. Chem. Soc. 1996, 118, 12854-12855. Enantioselective Protonation of Prochiral Silyl Enol Ethers and Ketene Silyl Acetals Ishihara, K.; Kaneeda, M.; Yamamoto, H. J. Am. Chem. Soc. 1994, 116, 11179-11180. Ishihara, K.; Nakamura, S.; Kaneeda, M.; Yamamoto, H. J. Am. Chem. Soc. 1996, 118, 12854-12855. Catalytic Enantioselective Protonation of Prochiral Silyl Enol Ethers Ishihara, K.; Nakamura, S.; Kaneeda, M.; Yamamoto, H. J. Am. Chem. Soc. 1996, 118, 12854-12855. Catalytic Enantioselective Protonation of Prochiral Silyl Enol Ethers Ishihara, K.; Nakamura, S.; Kaneeda, M.; Yamamoto, H. J. Am. Chem. Soc. 1996, 118, 12854-12855. Catalytic Enantioselective Protonation of Prochiral Silyl Ketene Acetals Ishihara, K.; Nakamura, S.; Kaneeda, M.; Yamamoto, H. J. Am. Chem. Soc. 1996, 118, 12854-12855. Catalytic Enantioselective Protonation of Prochiral Silyl Ketene Acetals Entry Chiral LBA (mol %) SnCl4 (mol %) Time (h) ee (%) 1 (R)-BINOL-OMe (2) 110 1 90 2 (R)-BINOL-OMe (5) 110 0.5 91 3 (R)-BINOL (5) 110 0.5 80 4 (R)-BINOL-OMe (2) 50 2 90 5 (R)-BINOL-OMe (20) 16 1 0 6 (R)-BINOL-OMe (100) 100 0.2 98 Ishihara, K.; Nakamura, S.; Kaneeda, M.; Yamamoto, H. J. Am. Chem. Soc. 1996, 118, 12854-12855. Catalytic Enantioselective Protonation of Prochiral Silyl Ketene Acetals Entry Chiral LBA (mol %) SnCl4 (mol %) Time (h) ee (%) 1 (R)-BINOL-OMe (2) 110 1 90 2 (R)-BINOL-OMe (5) 110 0.5 91 3 (R)-BINOL (5) 110 0.5 80 4 (R)-BINOL-OMe (2) 50 2 90 5 (R)-BINOL-OMe (20) 16 1 0 6 (R)-BINOL-OMe (100) 100 0.2 98 Ishihara, K.; Nakamura, S.; Kaneeda, M.; Yamamoto, H. J. Am. Chem. Soc. 1996, 118, 12854-12855. Catalytic Enantioselective Protonation of Prochiral Silyl Ketene Acetals • 97% Conversion with (R)-BINOL-OMe LBA vs 17% Conversion with phenol-LBA and 0% with SnCl4 (no acid present)! Ishihara, K.; Nakamura, S.; Kaneeda, M.; Yamamoto, H. J. Am. Chem. Soc. 1996, 118, 12854-12855. Recent Improvements with Chiral Brønsted Acids (Chiral N-Triflylthiophosphoramide) Cheon, C. H.; Yamamoto, H. J. Am. Chem. Soc. 2008, 130, 9246-9247. Recent Improvements with Chiral Brønsted Acids (Chiral N-Triflylthiophosphoramide) Cheon, C. H.; Yamamoto, H. J. Am. Chem. Soc. 2008, 130, 9246-9247. Recent Improvements with Chiral Brønsted Acids (Chiral N-Triflylthiophosphoramide) Cheon, C. H.; Yamamoto, H. J. Am. Chem. Soc. 2008, 130, 9246-9247. Recent Improvements with Chiral Chincona as Latent HF Source Poisson , T.; Dalla, V.; Marsais, F.; Dupas, G.; Oudeyer, S.; Levacher, V. Angew. Chem., Int. Ed. 2007, 46, 7090-7093. Recent Improvements with Chiral Chincona as Latent HF Source Poisson , T.; Dalla, V.; Marsais, F.; Dupas, G.; Oudeyer, S.; Levacher, V. Angew. Chem., Int. Ed. 2007, 46, 7090-7093. Chiral Guanidine Catalyzed Conjugate Addition/ Enantioselective Protonation Leow, D.; Lin, S.; Chittimalla, S. K.; Fu, X.; Tan, C.-H. Angew. Chem., Int. Ed. 2008, 47, 5641-5647. Chiral Guanidine Catalyzed Conjugate Addition/ Enantioselective Protonation Leow, D.; Lin, S.; Chittimalla, S. K.; Fu, X.; Tan, C.-H. Angew. Chem., Int. Ed. 2008, 47, 5641-5647. Rhodium Catalyzed Conjugate Addition/Enantioselective Protonation Navarre, L.; Darses, S.; Genet, J.-P. Angew. Chem., Int. Ed. 2004, 43, 719-723. Navarre, L.; Martinez, R.; Genet, J.-P.; Darses, S. J. Am. Chem. Soc. 2008, 130, 6159-6169. Rhodium Catalyzed Conjugate Addition/Enantioselective Protonation Navarre, L.; Darses, S.; Genet, J.-P. Angew. Chem., Int. Ed. 2004, 43, 719-723. Navarre, L.; Martinez, R.; Genet, J.-P.; Darses, S. J. Am. Chem. Soc. 2008, 130, 6159-6169. Rhodium Catalyzed Conjugate Addition/Enantioselective Protonation Entry PG R pKa of SM Yield (%) ee (%) pKa of Prod 1 Ac Me 13.1 91 90 14.7 2 CBz Me 9.4 92 43 11.0 3 Boc Me 9.6 82 90 11.2 4 Phth Me - 91 10 - 5 COCF3 Me 8.05 100 15 9.67 6 Ac i-Pr - 87 91 - 7 Boc i-Pr - 76 93 - 8 Boc t-Bu - 70 95 - Navarre, L.; Darses, S.; Genet, J.-P. Angew. Chem., Int. Ed. 2004, 43, 719-723. Navarre, L.; Martinez, R.; Genet, J.-P.; Darses, S. J. Am. Chem. Soc. 2008, 130, 6159-6169. Rhodium Catalyzed Conjugate Addition/Enantioselective Protonation Navarre, L.; Darses, S.; Genet, J.-P. Angew. Chem., Int. Ed. 2004, 43, 719-723. Navarre, L.; Martinez, R.; Genet, J.-P.; Darses, S. J. Am. Chem. Soc. 2008, 130, 6159-6169. Rhodium Catalyzed Conjugate Addition/Enantioselective Protonation Navarre, L.; Darses, S.; Genet, J.-P. Angew. Chem., Int. Ed. 2004, 43, 719-723. Navarre, L.; Martinez, R.; Genet, J.-P.; Darses, S. J. Am. Chem. Soc. 2008, 130, 6159-6169. Protonation by Chiral Brønsted Base – G. C. Fu Hodous, B. L.; Ruble, J. C.; Fu, G. C. J. Am. Chem. Soc. 1999, 121, 2637-2638. Wiskur, S. L.; Fu, G. C. J. Am. Chem. Soc. 2005, 127, 6176-6177. Protonation by Chiral Brønsted Base – G. C. Fu • ee% of product varies linearly with with ee% of starting catalyst Hodous, B. L.; Ruble, J. C.; Fu, G. C. J. Am. Chem. Soc. 1999, 121, 2637-2638. Wiskur, S. L.; Fu, G. C. J. Am. Chem. Soc. 2005, 127, 6176-6177. Protonation by Chiral Brønsted Base – G. C. Fu Hodous, B. L.; Ruble, J. C.; Fu, G. C. J. Am. Chem. Soc. 1999, 121, 2637-2638. M. Poirier Literature Meeting (Oct 2th 2007) Protonation by Chiral Brønsted Acid – G. C. Fu Hodous, B. L.; Ruble, J. C.; Fu, G. C. J. Am. Chem. Soc. 1999, 121, 2637-2638. Wiskur, S. L.; Fu, G. C. J. Am. Chem. Soc. 2005, 127, 6176-6177. Protonation by Chiral Brønsted Acid – G. C. Fu Hodous, B. L.; Ruble, J. C.; Fu, G. C. J. Am. Chem. Soc. 1999, 121, 2637-2638. Wiskur, S. L.; Fu, G. C. J. Am. Chem. Soc. 2005, 127, 6176-6177. Protonation by Palladium Mediated Decarboxylative Protonation – B. M. Stoltz Mohr, J. T.; Nishimata, T.; Behenna, D. C.; Stoltz, B. M. J. Am. Chem. Soc. 2006, 128, 11348-11349. Marinescu, S. C.; Nishimata, T.; Mohr, J. T.; Stoltz, B. M. Org. Lett. 2008, 10, 1039-1042. Behenna, D. C.; Stoltz, B. M. J. Am. Chem. Soc. 2004, 126, 15044-15045. Protonation by Palladium Mediated Decarboxylative Protonation – B. M. Stoltz Mohr, J. T.; Nishimata, T.; Behenna, D. C.; Stoltz, B. M. J. Am. Chem. Soc. 2006, 128, 11348-11349. Marinescu, S. C.; Nishimata, T.; Mohr, J. T.; Stoltz, B. M. Org. Lett. 2008, 10, 1039-1042. Behenna, D. C.; Stoltz, B. M. J. Am. Chem. Soc. 2004, 126, 15044-15045. Protonation by Palladium Mediated Decarboxylative Protonation – B. M. Stoltz Mohr, J. T.; Nishimata, T.; Behenna, D. C.; Stoltz, B. M. J. Am. Chem. Soc. 2006, 128, 11348-11349. Marinescu, S. C.; Nishimata, T.; Mohr, J. T.; Stoltz, B. M. Org. Lett. 2008, 10, 1039-1042. Protonation by Palladium Mediated Decarboxylative Protonation – B. M. Stoltz Mohr, J. T.; Nishimata, T.; Behenna, D. C.; Stoltz, B. M. J. Am. Chem. Soc. 2006, 128, 11348-11349. Marinescu, S. C.; Nishimata, T.; Mohr, J. T.; Stoltz, B. M. Org. Lett. 2008, 10, 1039-1042. Protonation by Palladium Mediated Decarboxylative Protonation – B. M. Stoltz • Excess of HCO2H led to decreased enantioselectivity, while smaller amounts of HCO2H increased allylation. • Small amount of 4Å MS decreased enantioselectivity, while large quantity increased allylation. • 5-8 equiv of HCO2H and 1.80g 4Å MS/mmol substrate was optimal… Mohr, J. T.; Nishimata, T.; Behenna, D. C.; Stoltz, B. M. J. Am. Chem. Soc. 2006, 128, 11348-11349. Marinescu, S. C.; Nishimata, T.; Mohr, J. T.; Stoltz, B. M. Org. Lett. 2008, 10, 1039-1042. Protonation by Palladium Mediated Decarboxylative Protonation – B. M. Stoltz Mohr, J. T.; Nishimata, T.; Behenna, D. C.; Stoltz, B. M. J. Am. Chem. Soc. 2006, 128, 11348-11349. Marinescu, S. C.; Nishimata, T.; Mohr, J. T.; Stoltz, B. M. Org. Lett. 2008, 10, 1039-1042. Protonation by Palladium Mediated Decarboxylative Protonation – B. M. Stoltz Mohr, J. T.; Nishimata, T.; Behenna, D. C.; Stoltz, B. M. J. Am. Chem. Soc. 2006, 128, 11348-11349. Marinescu, S. C.; Nishimata, T.; Mohr, J. T.; Stoltz, B. M. Org. Lett. 2008, 10, 1039-1042. Concluding Remarks – Take Home Message • A great deal of energy has been put to introduce a simple proton to form chiral enantioenriched tertiary carbon center • Although we have ennumerated numerous parameters that are critical to achieve high enantioselectivities, few mechanistic understanding of their behaviour are proposed yet. • ‘‘AP’’ can be used for making α- and β-amino acids and few natural products • Enantioselective protonnation should continue to rise as an important tool for understanding general organic chemistry Matoishi, K.; Ueda, M.; Miyamoto, M.; Ohta, H. J. Mol. Catal. B 2004, 27, 161-168. Blanchet, J.; Baudoux, J.; Amere, M.; Lasne, M.-C.; Rouden, J. Eur. J. Org. Chem. 2008, 5493-5506.








