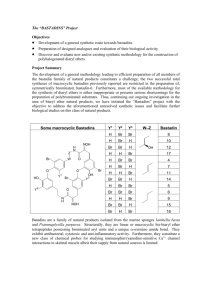
Polyketides: Conformation & Synthesis
advertisement

Recent Development for Stereoselective Synthesis of 1,3-Polyol Ye Zhu Prof. Burgess’ Group Aug. 19, 2010 Introduction The 1,3-polyol structure cluster is present in numerous natural products ranging from antibiotics to marine natural products. A substructure search with SciFinder gave 87 and 35 natural products bearing one or more of 1,3,5,7,9-pentaol(s) and 1,3,5,7,9,11,13-heptaol(s), respectively. Introduction Michael, Muller, et al, Synthesis, 2006, 557. Introduction C-C Bond Forming Reactions Asymmetric Alkylation Knochel, P., et al, Tetrahedron Lett., 1993, 5881. Introduction C-C Bond Forming Reactions Asymmetric Allylation advantage: allylation can be carried out iteratively easily Leighton, J.L. et al, Angew. Chem. Int. Ed. 2003, 946. Introduction C-C Bond Forming Reactions Reagent Controlled Aldol Reaction Mahler, U., et al, Chem. Ber. 1988, 2035. Introduction Stereoselective Reduction Directed Reduction of β-Hydroxyketones Introduction Stereoselective Reduction Asymmetric Hydrogenation Uchida Y.. et al, Tetrahedron 1993, 1997. Introduction Homoallylic Alcohol As Substrate Asymmetric Silylformylation Leighton, J.L. et al, J. Am. Chem. Soc. 2000, 122, 8587. Catalytic Asymmetric Overman Esterification Overman, L.E.; et al, J. Am. Chem. Soc. 2005, 2866. Kirsch, S.F., et al, Chem. Commun.., 2007, 4164. Kirsch, S.F., et al, Chem. Commun.., 2007, 4164. Kirsch, S.F., et al, Org. Lett., 2009, 5634. 1,3-anti-Diols Through Catalytic Hydrogenations Genet J.P., et al, Org. Lett., 2007, 105. Iterative Cr-Mediated Catalytic Asymmetric Allylation Kishi, Y., et al, Org. Lett., 2008, 3073. Kishi, Y., et al, Org. Lett., 2008, 3077. Iterative Cr-Mediated Catalytic Asymmetric Allylation Kishi, Y., et al, Org. Lett., 2008, 3073. Kishi, Y., et al, Org. Lett., 2008, 3077. Proline-Catalyzed Sequential αAminoxylation and Horner-WadsworthEmmons Olefination of Aldehydes Kumar, P., et al, Org. Lett., 2009, 2611. Iridium-Catalyzed Enantioselective Carbonyl Allylation and Iterative TwoDirectional Assembly of 1,3-Polyols Krische, M. J. et al, Angew. Chem. Int. Ed., 2009, 5018. Elongation of 1,3-Polyols via Iterative Catalyst-Directed Carbonyl Allylation from the Alcohol Oxidation Level Krische, M. J. et al, Org. Lett., 2009, 3112. Elongation of 1,3-Polyols via Iterative Catalyst-Directed Carbonyl Allylation from the Alcohol Oxidation Level Krische, M. J. et al, Org. Lett., 2009, 3112. Elongation of 1,3-Polyols via Iterative Catalyst-Directed Carbonyl Allylation from the Alcohol Oxidation Level Iridium-Catalyzed Enantioselective Carbonyl Allylation and Iterative TwoDirectional Assembly of 1,3-Polyols Krische, M. J. et al, Angew. Chem. Int. Ed., 2009, 5018. Iridium-Catalyzed Enantioselective Carbonyl Allylation and Iterative TwoDirectional Assembly of 1,3-Polyols Krische, M. J. et al, Angew. Chem. Int. Ed., 2009, 5018. Direct Catalytic Asymmetric Aldol Reactions of Thioamides: Toward a Stereocontrolled Synthesis of 1,3-Polyols Shibasaki, M. et al, J. Am. Chem.Soc., 2009, 18244. Direct Catalytic Asymmetric Aldol Reactions of Thioamides: Toward a Stereocontrolled Synthesis of 1,3-Polyols Shibasaki, M. et al, J. Am. Chem.Soc., 2009, 18244. STEREOCHEMISTRY OF ALTERNATING POLYOL CHAINS: 13C NMR ANALYSIS OF 1,3-DIOL ACETONIDES Rycbnovsky, S.D., et al, Tetrahedron Lett., 1990, 945 Rycbnovsky, S.D., et al, Tetrahedron Lett., 1990, 945 Thanks