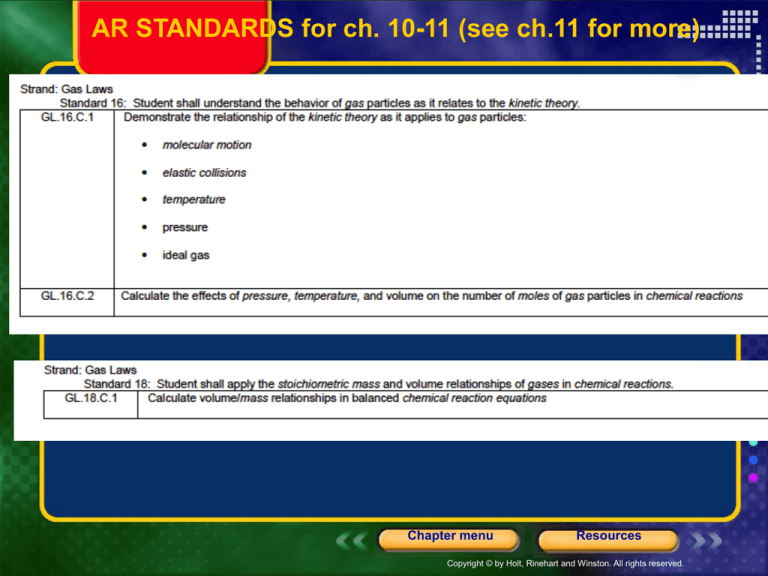
AR STANDARDS for ch. 10-11 (see ch.11 for more)
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 10
States of Matter
Table of Contents
Section 1 The Kinetic-Molecular Theory of Matter
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 10
Section 1 The Kinetic-Molecular
Theory of Matter
Objectives
• State the kinetic-molecular theory of matter, and
describe how it explains certain properties of matter.
• List
theoffive
assumptions
of terms
the kinetic-molecular
theory
gases.
Define
the
ideal gas and real
gas.
• Describe
each
of theexpansion,
following density,
characteristic
properties
of
gases:
compressibility, diffusion, and effusion. fluidity,
• Describe
the conditions
under which a real gas
deviates from
“ideal” behavior.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
10.1
The Nature of Gases
The skunk releases its spray!
Within seconds you smell that
all-too-familiar foul odor. You will
discover some general
characteristics of gases that help
explain how odors travel through
the air, even on a windless day.
Slide
of 29
4
© Copyright Pearson Prentice Hall
End Show
10.1
The Nature of Gases
>
Kinetic Theory and a Model for Gases
The word kinetic refers to motion.
• The energy an object has because of its
motion is called kinetic energy.
• According to the kinetic theory, all matter
consists of tiny particles that are in constant
motion.
Slide
of 29
5
© Copyright Pearson Prentice Hall
End Show
Chapter 10
Section 1 The Kinetic-Molecular
Theory of Matter
• The kinetic-molecular theory is based on the idea
that particles of matter are always in motion.
• The theory can be used to explain the properties of
solids, liquids, and gases in terms of the energy of
particles and the forces that act between them.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
10.1
The Nature of Gases
>
Kinetic Theory and a Model for Gases
Kinetic Theory and a Model for Gases
What are the assumptions of the kinetic
theory as it applies to gases?
Slide
of 29
7
© Copyright Pearson Prentice Hall
End Show
10.1
The Nature of Gases
>
Kinetic Theory and a Model for
Gases
According to kinetic theory:
• The particles in a gas are considered
to be small, hard spheres with an
insignificant volume.
• The motion of the particles in a gas is
rapid, constant, and random.
• All collisions between particles in a
gas are perfectly elastic.
Slide
of 29
8
© Copyright Pearson Prentice Hall
End Show
Section 1 The Kinetic-Molecular
Theory of Matter
Chapter 10
The Kinetic-Molecular Theory of Gases
• An ideal gas is a hypothetical gas that perfectly fits all the
assumptions of the kinetic-molecular theory.
• The kinetic-molecular theory of gases is based on the
following five assumptions:
• Gases consist of large numbers of tiny particles that are
far apart relative to their size.
• Most
of
volume
occupied
by lower
a gasdensity
is empty
space
which
is the
the
reason
that
gases
have
than
liquids
and solids
and
are easily
compressed.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 10
Section 1 The Kinetic-Molecular
Theory of Matter
The Kinetic-Molecular Theory of Gases,
continued
• Collisions between gas particles and between
particles and container walls are elastic collisions.
• An
is one in which there is no net
losselastic
of totalcollision
kinetic energy.
• Gas particles are in continuous, rapid, random
motion. They therefore possess kinetic energy, which
is energy of motion.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 10
Section 1 The Kinetic-Molecular
Theory of Matter
The Kinetic-Molecular Theory of Gases,
continued
• There are no forces of attraction between gas
particles.
4. The temperature of a gas depends on the average
kinetic energy of the particles of the gas.
• The kinetic energy of any moving object is given by
the following equation:
1
KE m2
2
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 10
Section 1 The Kinetic-Molecular
Theory of Matter
The Kinetic-Molecular Theory of Gases,
continued
• All gases at the same temperature have the same
average kinetic energy.
• At the same temperature, lighter gas particles, have
higher average speeds than do heavier gas particles.
• Hydrogen molecules will have a higher speed than
oxygen molecules.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 10
Visual Concepts
Kinetic Molecular Theory
Click below to watch the Visual Concept.
http://my.hrw.com/sh/hc6_003036809x/stu
Visual Concept
dent/ch10/sec01/vc00/hc610_01_v00fs.ht
m
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
10.1
The Nature of Gases
>
Kinetic Theory and a Model for Gases
Properties of Gases
Particles in a gas are in rapid, constant motion.
Slide
of 29
14
© Copyright Pearson Prentice Hall
End Show
10.1
The Nature of Gases
>
Kinetic Theory and a Model for Gases
Properties of Gases
Gas particles travel in straight-line paths.
Slide
of 29
15
© Copyright Pearson Prentice Hall
End Show
10.1
The Nature of Gases
>
Kinetic Theory and a Model for Gases
Properties of Gases
The gas fills the container.
Slide
of 29
16
© Copyright Pearson Prentice Hall
End Show
Chapter 10
Visual Concepts
Additional Properties of Gases - watch visual
Click below to watch the Visual Concept.
http://my.hrw.com/sh/hc6_003036809x
Visual Concept
/student/ch10/sec01/vc03/hc610_01_v
03fs.htm
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 10
Section 1 The Kinetic-Molecular
Theory of Matter
The Kinetic-Molecular Theory and the Nature of
Gases
• The kinetic-molecular theory applies only to ideal
gases.
• Many gases behave nearly ideally if pressure is not
very high and temperature is not very low.
Expansion
• Gases do not have a definite shape or a definite
volume.
• They completely fill any container in which they are
enclosed.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 10
Section 1 The Kinetic-Molecular
Theory of Matter
The Kinetic-Molecular Theory and the Nature of
Gases, continued
Expansion, continued
• Gas particles move rapidly in all directions
(assumption 3) without significant attraction between
them (assumption 4).
Fluidity
• Because the attractive forces between gas particles
are insignificant (assumption 4), gas particles glide
easily past one another.
• Because liquids and gases flow, they are both referred
to as fluids.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Visual Concepts
Chapter 10
Fluid
QuickTime™ and a
Sorenson Video 3 decompressor
are needed to see this picture.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 10
Section 1 The Kinetic-Molecular
Theory of Matter
The Kinetic-Molecular Theory and the Nature of
Gases, continued
Low Density
• The density of a gaseous substance at atmospheric
pressure is about 1/1000 the density of the same
substance in the liquid or solid state.
• The reason is that the particles are so much farther
apart in the gaseous state (assumption 1).
Compressibility
• During compression, the gas particles, which are
initially very far apart (assumption 1), are crowded
closer together.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 10
Section 1 The Kinetic-Molecular
Theory of Matter
The Kinetic-Molecular Theory and the Nature of
Gases, continued
Diffusion and Effusion
• Gases spread out and mix with one another, even
without being stirred.
• The random and continuous motion of the gas
molecules (assumption 3) carries them throughout the
available space.
• Such spontaneous mixing of the particles of two
substances caused by their random motion is called
diffusion.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 10
Section 1 The Kinetic-Molecular
Theory of Matter
The Kinetic-Molecular Theory and the Nature of
Gases, continued
Diffusion and Effusion, continued
• Effusion is a process by which gas particles pass
through a tiny opening.
• The rates of effusion of different gases are directly
proportional to the velocities of their particles.
• Molecules of low mass effuse faster than molecules of
high mass.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 10
Visual Concepts
Comparing Diffusion and Effusion
Click below to watch the Visual Concept.
http://my.hrw.com/sh/hc6_003036809x/st
Visual Concept
udent/ch10/sec01/vc05/hc610_01_v05fs.
htm
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 10
Section 1 The Kinetic-Molecular
Theory of Matter
Deviations of Real Gases from Ideal Behavior
• Because particles of gases occupy space and exert
attractive forces on each other, all real gases deviate to
some degree from ideal gas behavior.
• A
real gasto
is the
a gas
that does not
behave
completely
according
assumptions
of
the
kinetic-molecular
theory.
• At
very
hightopressures
anda low
temperatures,
a gas is
most
likely
behave like
nonideal
gas.
• The
polar
molecules
a gas
the
moremore
the gas
willthe
deviate
from of
ideal
gasare,
behavior.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
10.1
The Nature of Gases
>
Gas Pressure
Gas Pressure
How does kinetic theory explain
gas pressure?
Slide
of 29
26
© Copyright Pearson Prentice Hall
End Show
10.1
The Nature of Gases
>
Gas Pressure
Gas pressure results from the force exerted by
a gas per unit surface area of an object.
• An empty space with no particles and no
pressure is called a vacuum.
• Atmospheric pressure results from the
collisions of atoms and molecules in air with
objects.
Slide
of 29
27
© Copyright Pearson Prentice Hall
End Show
10.1
The Nature of Gases
>
Gas Pressure
Gas pressure is the result of
simultaneous collisions of billions of
rapidly moving particles in a gas with
an object.
Slide
of 29
28
© Copyright Pearson Prentice Hall
End Show
10.1
The Nature of Gases
>
Gas Pressure
A barometer is a device that is used to measure
atmospheric pressure.
Slide
of 29
29
© Copyright Pearson Prentice Hall
End Show
10.1
The Nature of Gases
>
Gas Pressure
• The SI unit of pressure is the pascal (Pa).
• One standard atmosphere (atm) is the
pressure required to support 760 mm of
mercury in a mercury barometer at 25°C.
Slide
of 29
30
© Copyright Pearson Prentice Hall
End Show
SAMPLE PROBLEM 13.1
Slide
of 29
© Copyright Pearson Prentice Hall
End Show
SAMPLE PROBLEM 13.1
Slide
of 29
© Copyright Pearson Prentice Hall
End Show
SAMPLE PROBLEM 13.1
Slide
of 29
© Copyright Pearson Prentice Hall
End Show
SAMPLE PROBLEM 13.1
Slide
of 29
© Copyright Pearson Prentice Hall
End Show
Practice Problems for Sample Problem 13.1
Problem Solving 13.1 Solve
Problem 1 with the help of an
interactive guided tutorial.
Slide
of 29
© Copyright Pearson Prentice Hall
End Show
10.1
The Nature of Gases
>
Kinetic Energy and Temperature
Kinetic Energy and Temperature
What is the relationship between the
temperature in kelvins and the average
kinetic energy of particles?
Slide
of 29
36
© Copyright Pearson Prentice Hall
End Show
10.1
The Nature of Gases
>
Kinetic Energy and Temperature
Average Kinetic Energy
The particles in any collection of atoms or
molecules at a given temperature have a wide
range of kinetic energies. Most of the particles
have kinetic energies somewhere in the middle
of this range.
Slide
of 29
37
© Copyright Pearson Prentice Hall
End Show
10.1
The Nature of Gases
>
Kinetic Energy and Temperature
Slide
of 29
38
© Copyright Pearson Prentice Hall
End Show
10.1
The Nature of Gases
>
Kinetic Energy and Temperature
Absolute zero (0 K, or –273.15°C) is the
temperature at which the motion of particles
theoretically ceases.
• Particles would have no kinetic energy at
absolute zero.
• Absolute zero has never been produced in
the laboratory.
Slide
of 29
39
© Copyright Pearson Prentice Hall
End Show
10.1
The Nature of Gases
>
Kinetic Energy and Temperature
Average Kinetic Energy and Kelvin Temperature
The Kelvin temperature of a substance
is directly proportional to the average
kinetic energy10.1
of the particles of the
substance.
Slide
of 29
40
© Copyright Pearson Prentice Hall
End Show
10.1
The Nature of Gases
>
Kinetic Energy and Temperature
In this vacuum chamber, scientists cooled
sodium vapor to nearly absolute zero.
Slide
of 29
41
© Copyright Pearson Prentice Hall
End Show
The Nature of Gases
>
Kinetic Energy and Temperature
Animation 14
Observe particles in motion and discover
the connection between temperature and
kinetic energy.
Slide
of 29
42
© Copyright Pearson Prentice Hall
End Show
10.1 Section Quiz.
Assess students’ understanding of
the concepts in Section
10.1.
Continue to:
-or-
Launch:
Section Quiz
Slide
of 29
© Copyright Pearson Prentice Hall
End Show
10.1 Section Quiz.
1. According to the kinetic theory, the particles in
a gas
a. are attracted to each other.
b. are in constant random motion.
c. have the same kinetic energy.
d. have a significant volume.
Slide
of 29
© Copyright Pearson Prentice Hall
End Show
10.1 Section Quiz.
2. The pressure a gas exerts on another object
is caused by
a. the physical size of the gas particles.
b. collisions between gas particles and the
object.
c. collisions between gas particles.
d. the chemical composition of the gas.
Slide
of 29
© Copyright Pearson Prentice Hall
End Show
10.1 Section Quiz.
3. The average kinetic energy of the particles in
a substance is directly proportional to the
a. Fahrenheit temperature.
b. Kelvin temperature.
c. molar mass of the substance.
d. Celsius temperature.
Slide
of 29
© Copyright Pearson Prentice Hall
End Show
Online Self-Check Quiz
Complete the online Quiz and record answers.
Ask if you have any questions about your
answers.
click here for online Quiz 10.1
(8 questions)
You must be in the “Play mode” for the
slideshow for hyperlink to work.
Slide
of 25
© Copyright Pearson Prentice Hall
End Show
VIDEOS FOR ADDITIONAL INSTRUCTION
Additional Videos for
Chapter 10: States of Matter
Section 10.1: Kinetic Theory of Matter
•Gases
•Ideal Gas Law
•Three States of Matter
Slide
of 28
© Copyright Pearson Prentice Hall
End Show
End of Chapter 10.1 Show
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.








