from 1,5 – dicarbonyl compounds and ammonia
advertisement
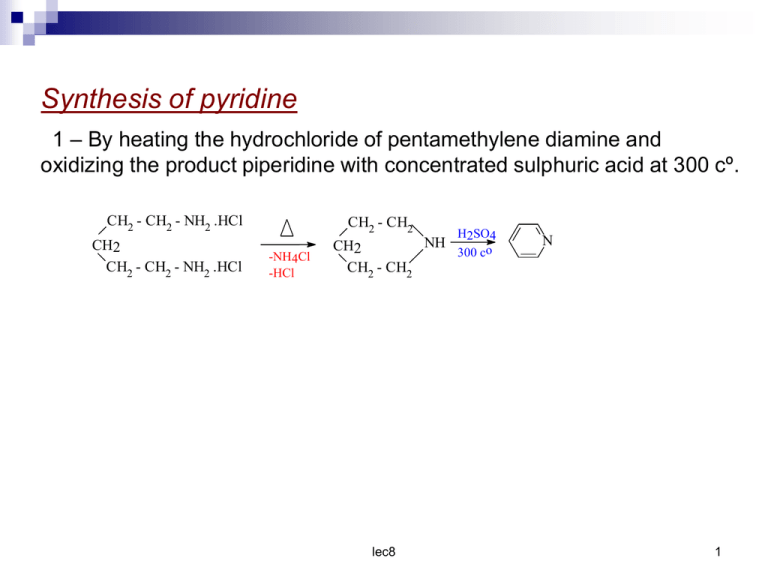
Synthesis of pyridine 1 – By heating the hydrochloride of pentamethylene diamine and oxidizing the product piperidine with concentrated sulphuric acid at 300 cº. CH2 - CH2 - NH2 .HCl CH2 CH2 - CH2 - NH2 .HCl CH2 - CH2 -NH4Cl -HCl H2SO4 NH CH2 300 co CH2 - CH2 lec8 N 1 2 – from 1,5 – dicarbonyl compounds and ammonia : Ammonia react with 1,5 – dicarbonyl compounds to give 1,4 dihydropyridine which are easily dehydrogenated to pyridines .The reaction proceed via loss of two molecules of water . H H H H H + O O N-H H -2H2O Oxidation N H 1,4 - dihydropyridine lec8 N Pyridine 2 3- From ethyl acetoacetate two mole with dichloromethane in presences of ammonia . EtOOC CH2 | C=O CH3 EtOOC CH2 | O=C CH3 NaOEt CH2Cl2 EtOOC H - EtOOC || C CH3 OH CH2 EtOOC - C HC-COOEt || C CH3 OH CH3 EtOOC - C C-COOEt -H2O CH3 Oxide NH2 HO CH3 HO CH3 NH3 -H2O H EtOOC - C CH3 EtOOC - C CH3 HO H CH2 C-COOEt C-COOEt N H CH3 C-COOEt N CH3 lec8 3 4 – From 1,3 – dicarbonyl compound and cyanoacetamide CH3 C=O | CH2 O=C | CH3 CH3 CN + H H C CH2 C H C=O CH3 CN C=O C =O H2N H2N acetylacetone OH C cyanoacetamide H CH CH H C - CN CH3 -C OH C C CN -2H2O C=O CH3 N H H C C C =O OH H2N CH CH C - CN CH3 -C C - OH N lec8 4 Chemical reactions Basicity of pyridine Pyridine behaves as abase , It react with acids to from fairly stable salt . The reason for the basic character of pyridine is that the nitrogen lone pair being in sp2 hybrid orbital is not involve in the delocalized π molecular orbital .It is readily available for the formation of a new p N – H bond with proton . Pyridine is a stronger base than pyrrole in which the basicity is reduced by delocalization of the nitrogen lone pair Pyrrole < Pyridine < aliphatic amine N H RNH2 N Basicity increase lec8 5 Addition and ring – opening reaction The acid derivatives combine with the pyridine to give a quaternary salt (e.g 2) .Which have been isolated as acidchlorides .This salt react with hydroxyl group yielding the Acyl derivative ; the liberated acid is taken up as the pyridine salt (3) .Quaternary salts as (2) are immediately decomposed by water to pyridine hydrochloride and the organic acid but with etheylcyanoacetate the ring open yielding (4) PhCoCl + N CoPh (2) N EtCOOCH2CN + Cl- R /OH PhCOOR + + N H (3) Cl- H2O + C(CN)CO2Et N NH | Ph - C = C(CN)CO2Et (4) lec8 Cl- PhCOOH 6 The ring is comparatively easily opened by nucleophilic reagent 2,4 – Dinitrophenylpyridinum chloride (5) is a colourless crystalline solid which is formed from pyridine and 2,4 – dinitrochlorobenzene at 100 cº this reaction is reversed at 200 cº with water at 150 cº yield pyridine hydrochloride and 2,4 – dinitrophenol , but with cold aqueous alkali a deep red compound (6) is formed which on successive treatment with dilute aniline and acid yields 2,4 – dinitroaniline and glutaconic aldehyde dianil (7) . lec8 7 NO2 100 c0 + N Cl 200 c0 NO2 Cl- N NO2 2.4 - dinitrophenylpyridinum chlorid cold aquKOH NO2 H C (5) CH CH HO - CH CH PhNH2 N PhNH2 NO2 + N H + KCl NO2 (6) 150 c0 H 2O + OH H C OH NO2 CH + Ph - NH - CH Pridinium Chloride NO2 aniline Acid CH Cl- NO2 2.4 - dinitrophenol CH = NPh (7) glutaconic aldehydedianil NO2 lec8 8 Reduction Pyridine is easily reduce to hexahydropyridin or piperidine by a variety of method including hydrogen over Raney nickel , rubidium at 60 ºc palladium charcoal with acetic acid .1,4 – dihydropyridine has however been obtained by the reduction of pyridine with trimethylsilane . N + Me3Si H Pd catalyst N SiMe3 0.1% KOH MeOH N H The pyridine is cleaved by ultrasonic waves giving acetylene and hydrogen cyanide . N ultra sound 2 CH lec8 CH + HCN 9 Electrophilic subistitution reactions . Toward electrophilic subistitution pyridine resembles a deactivated benzene derivative it is often compared to nitrobenzene in reactivity (+) N (+) N (+) N N (+) N = O O (+) (+) N N O O O O (-) (-) lec8 N O O (-) 10 when the reaction take place the attack at β position can be understood in term of the resonance forms shown above in which ( α ) and ( γ ) position have a positive charge , this orientation can be understood also by comparison of the intermediate resulting from attack at various position H H H E N .. H ..N E H ..N E H Attack at β position H E N .. H H H E H ..N E N .. Attack at γ position lec8 11 three resonance can be written for each intermediate but one of these in the case of attack at the γ ( or α ) leaves positive charge on nitrogen this must be regarded as an unfavorable structure as compared to one in which carbon has a positive charge for nitrogen more electronegative than carbon . NO2 KNO3 H2SO4 N 3 - nitropyridine 370 c0 N SO3H HgSO4 H2SO4 N Br2 300 c0 3 - pyridinesulphonicacid Br N Rx or RCox AlCl3 3 - bromo pyridine No reaction lec8 12 Nucleophilic subistitution in pyridine The reactivity of pyridine toward nucleophilic subistitution is so great even the powerfully basic hydride ion , H- , can be displaced .Two important example of this reaction are amination by sodium amide and Alkylation by organolithium compounds . + - + NaNH2 heat N H N + NH3 NH2 N Na - + NaNH2 NH2 + NH3 NHNa Sod salt of 2 - amino pyriine N + - + C6H5Li heat N H N Ph + LiH N Li Ph 2 - phenylpyridine KOH N 320 N OH N H O The attack take place at α – position because the positive charge arises in α – position lec8 13 Pyrylium salt and pyrans Very few simple derivatives of the aromatic pyrylium cation (1) are known , although benzopyrylium are widely distributed as flower petal colouring matters .The potentially very reactive 4-pyran (3) has been obtained recently and the pyranes ( 4 and 5 ) are well known .2,3 – Dihydro – 4 – pyran (6) has received some attention now it is easily available , and tetrahydropyran ( 7 ) is used as a synthetic intermediate .The sulphur analogues of these compounds have received little attention until very recently O 4 5 6 4 3 + O 3 2 O 1 O O 2 O O 1 1 2 Pyriliumcation 5 O 5 6 3 4 5 6 O 7 2pyrone 4 pyrone 4 3 6 + 7 8 O 2 1 lec8 14 Pyrylium salts The sodium salt of glutaconic aldehyde with perchloric acid at – 20 cº gives ared oxonium salt , which on standing at o cº cyclizes to the colourless pyrylium perchlorate (8) .This perchlorate has received little attention ,but with ammonia it yields 2,4,6 – triphenylpyrylium ferrichloride ( 9 ) can be prepared easily . It is stable in acid solution and nitrates . with ammonia it yields 2,4,6 – triphenylpyridine while with alkali the ring is opened , yielding (II) through the intermediate (10 ) - + CH CH CH CH = O Na O CH CH H ClO 4 O2- HO 6 4 O CH + CH = OH CH c0 0 -H2O - ClO4 sod . salt of glutaconic aldehyde Oxonium salt 5 CH 3 2 - ClO4 1 )8( Pyrylium perchlorate lec8 15 + CH Ph CH H CH2 CH2 FeCl3 O = C-Ph Ph -C = O Ph CH CH2 C= O Ph C=O Ph phenyl 1 - phenyl 2 - benzoyl ethylene methyl ketone Ph Ph - FeCl4 + Ph O Ph NH3 (A) Ph N Ph 2,4,6 - triphenyl Pyrylium ferricchloride NaHCO3 aq NaOAC orr (B) Ph CH Ph CH Ph O Ph OH CH2 C = O OC Ph lec8 Ph 16 Mechanism Ph CH Ph CH H + - CH + - CH2 Ph -C = O O = C-Ph CH2 FeCl 3 Ph C=O CH2 C= O Ph keto form Ph H C HC CH Ph C -H2O Ph enol form Ph CH Ph CH HC Ph CH + CH C - OH Ph HO -C Ph C O C CH Ph CH C - Ph FeCl4- )A ( HC NH 3 C O Ph H NH lec8 CH + C - Ph O 2 - 17 Ph CH HC Ph CH CH HC Ph CH + Ph C C - Ph HO N: H2 C Ph H + N -H2O C - Ph OH Ph H + N H Ph Ph Ph Ph N Ph Ph Ph B NaOaC Ph NaHCO3 aq + O Ph H+/ OH - + Ph O Ph Ph FeCl4- Ph O Ph OH Ph Ph Ph Ph O - + Ph OH Ph O Ph OH enol form OO Ph keto form 1,3,5 - triphenyl glutaconicdialdehyde lec8 18 Reaction of pyrylium cation A–Reaction with electrophilic reagent 2,4,6 –Triphenylpyrylium undergoes exchange at 3 – and 5 – position in hot deuteroacetic acid Ph Ph Ph ACDO + Ph O Ph -) ( + ACO OAC Ph O Ph D + Ph 5 + O D H OAC Ph B – No nitration of pyrylium are known lec8 19 Synthesis of α – pyrones 6 – hydroxyl – α – pyron can be prepared by heating a glutaconic acid with acetic anhydride . CH H2C O=C CH C=O OH OH - H 2O ( CH3CO) 2O lec8 HO O O 20 Synthesis of γ – Pyrones 1- γ – pyrone may be prepared by heating chelidonic acid just above its melting point .chelidonic acid may be prepared from acetone and ethyl oxalate CH3 + 2 (COOEt )2 O=C CH3 C2H5ONa CH2CO . COOEt O=C -2(EtOH) CH2CO . COOEt OH CH = C- COOEt CH = C- COOEt heat O=C O=C CH = C - OH COOEt O CH = C COOEt chelidonic acid O heat O γ - Pyrone lec8 21 2 – The dimethyl γ – pyrone may be prepared from the copper salt of ethylacetoacetate as follows CH3 -CO - CH - COOEt Cu CH3 -CO - CH - COOEt CoCl2 C=O CH3 -CO - CH - COOEt CH3 -CO - CH - COOEt CH3 -CO - CH2 hyd heat CH3 -C = CH OH C=O OH CH3 -C = CH CH3 -CO - CH2 CH3 heat -H2 O C C=O CH O C=O C CH CH3 lec8 22 Reaction of α and γ – Pyrone . 1- NaOH O HCl COONa ONa O COOH OH α - pyrone O 2- 3- O O NaOH H+ O O O + NH2NH2 OH OH O O -H2O O OH O OH NH NH | | NH2 NH 2 lec8 N O | NH2 1 - amino pyridone 23 O O O NH3 5- O CH3 CH3 -H2O CH3 OH CH3 CH3 NH2 R N H CH3 2,6 - di methyl - 4 - pyrone R R 6- CH3 + CH3MgBr O O R O OH O HO 7CH3 O + CH3MgBr CH3 CH3 .. O CH3 CH3 CH3 HClO4 -H2O + O CH3 CH3 ClO4- pyrylium salt O OCH3 CH3I 8CH3 O .. CH3 + CH3 O OCH3 I- HClO4 + CH3 -H I CH3 O CH3 ClO4lec8 24 R R P2S5 9R O O R O O S S P2S5 10 O R R R O R R R Br Br2 / ACOH 11 R O O R O O 3 -bromo - 2 - pyrone O O Br 12 CH3 Br 2 / ACOH O CH3 CH3 O CH3 3 -bromo - 4 - pyrone lec8 25 R R NO2 HNO3 / ACOH 13 R O O R O O O NO2 14 CH3 O HNO3 / ACOH O CH3 CH3 O CH3 3 -bromo - 4 - pyrone lec8 26 Sulphur – containing analogues 4 H – Thiopyran ( 1 ) has been obtained in asimilar way to its oxygen analogue and has b.p 30 cº at 12 mm it is readily oxidized by chlorine to thiapyrylium (2) chloride , and an alternative way of making this class of compound is outlined 2,4,6 triphenylthia pyrylium perchlorate (3) with phenyllithum gives the deep purple 1,2,4,6 – tetraphenylthia (IV) benzene (4) . On standing it isomerizes to colurless 4H - thiapyran (5) PhNMe2 Cl S Cl Cl2 40 S + S (1) (2) Ph Ph + S (3) Ph ClO4- PhLi Ph Ph isomerize Ph S Ph Ph (4) lec8 Ph S Ph (5) 27 1- methyl – 3,5 – diphenylthia(IV)benzene 1- oxide (6) has been obtained as indicated below .It has m.p 148º and can be sublimed at near this temperature at 0 – 0.5 mm pressure .The compound is therefore very much more stable than (IV) benzene such as (4) Ph - Ph O + S Me (6) lec8 28