Fullerene
advertisement

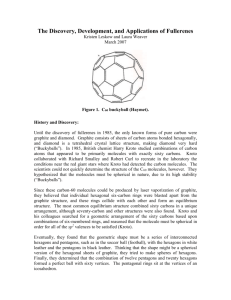
Nanotechnology Lecture 2 Fullerene Definition 1nm=10-9m Human Red Blood Cells=8000nm There is Plenty of Room at the Bottom (1918~ 1988) R.P.Feynman The Origin and Development of Nanotechnology • Feynman——《There is Plenty of Room at the Bottom 》 • The Invention of the Scanning Tunneling Microscope(STM) • The Nanotechnology Developed Rapidly in1990s, and the New Words Came Fast • One of the 9 Major Key Technologies in the Future Global Technology Development Why?--a materials perspective “quantum size effect”: dominant when the nanometer size range is reached. a number of physical (mechanical, electrical, optical, etc.) properties change when compared to macroscopic systems. For example: opaque substances become transparent (copper); stable materials turn combustible (aluminum); insoluble materials become soluble (gold). A material such as gold, which is chemically inert at normal scales, can serve as a potent chemical catalyst at nanoscales. Image of reconstruction on a clean Gold(100) surface, as visualized using scanning tunneling microscopy. The positions of the individual atoms composing the surface are visible Why?--Simple to complex: a molecular perspective •Molecular self-assembly: automatically arrange themselves into some useful conformation. • through a bottom-up approach to prepare small molecules to almost any structure The DNA structure at left (schematic shown) will self-assemble into the structure visualized by atomic force microscopy at right. Image from Strong How?--Tools and techniques •Tools: The atomic force microscope (AFM) and the Scanning Tunneling Microscope (STM) are two early versions of scanning probes that launched nanotechnology •They can be used to look at surfaces and to move atoms around. By designing different tips for these microscopes, they can be used for carving out structures on surfaces and to help guide self-assembling structures. Typical AFM setup. A microfabricated cantilever with a sharp tip is deflected by features on a sample surface, much like in a phonograph but on a much smaller scale. A laser beam reflects off the backside of the cantilever into a set of photodetectors, allowing the deflection to be measured and assembled into an image of the surface Bottom-up approaches •These seek to arrange smaller components into more complex assemblies •Molecular self-assembly seeks to use concepts of supremolecular chemistry, and molecular recognition in particular, to cause single-molecule components to automatically arrange themselves into some useful conformation. Sarfus image of a DNA biochip elaborated by bottom-up approach. Top-down approaches •These seek to create smaller devices by using larger ones to direct their assembly. •Solid-state silicon methods for fabricating microprocessors •Solid-state techniques can be used to create devices known as nanoelectromechanical systems or NEMS, which are related to microelectromechanical systems or MEMS. This device transfers energy from nano-thin layers of quantum wells to nanocrystals above them, causing the nanocrystals to emit visible light.[11] The Self-Cleanliness on the Surface of Lotus Flower Gooses and Ducks Keep Dryness under the Water No Washing Nano Suit Fullerene • • • • • • Introduction of Fullerene family (what is?) Fullerene (discovery, naming) Types of Fullerene and related structures Properties Synthesis (to fabricate) Potential and current applications What is Fullerene? A Fullerene is any molecule composed entirely of carbon, in the form of a hollow sphere, ellipsoid, or tube. Spherical fullerenes are also called buckyballs, and cylindrical ones are called carbon nanotubes or buckytubes. C60 in solution Buckminster fullerene C60 (left) and carbon nanotubes (right) are two examples of structures in the fullerene family. Allotropes of carbon Allotropes : different structural modifications of an element; the atoms of the element are bonded together in a different manner. Allotropes of carbon: •Diamond (hardest natural mineral) •Graphite(dry lubricant, graphene) •Amorphous carbon (coal and soot) •Fullerene family (C60, carbon nanotubes etc) Diamond and graphite are two allotropes of carbon: pure forms of the same element that differ in structure. Fullerene-History •The existence of C60 was predicted by Eiji Osawa of Toyohashi University of Technology in a Japanese magazine in 1970 •With mass spectrometry, discrete peaks were observed corresponding to molecules with the exact mass of sixty or seventy or more carbon atoms. In 1985, Harold Kroto etc , discovered C60, and shortly thereafter came to discover the fullerenes. Kroto, Curl, and Smalley were awarded the 1996 Nobel Prize in Chemistry for their roles in the discovery of this class of compounds. •Minute quantities of the fullerenes, in the form of C60, C70, C76, and C84 molecules, are produced in nature, hidden in soot and formed by lightning discharges in the atmosphere. Buckminsterfullerene C60 The Icosahedral Fullerene C540 Discovery of Carbon C60 • 1985, Robert F. Curl, … discovered a new form of carbon,that 60 or 70 carbon atoms could cluster together to form a cage-like molecule. • The molecular structure resembled the pattern of a soccer ball or the geodesic designs of Buckminster Fullerenes. Thus the name buckyballs or fullerenes. • Since then the discovery has led to new research in polymers, semiconductors, and other various areas. • Nobel Prize to their finders in 1996 Existence in the nature •In 1992, fullerenes were found in a family of minerals known as Shungites in Karelia, Russia. •In 2010, fullerenes (C60) have been discovered in a cloud of cosmic dust surrounding a distant star 6500 light years away. Using NASA's Spitzer infrared telescope the scientists spotted the molecules' unmistakable infrared signature. Sir Harry Kroto, who shared the 1996 Nobel Prize in Chemistry for the discovery of buckyballs commented: "This most exciting breakthrough provides convincing evidence that the buckyball has, as I long suspected, existed since time immemorial in the dark recesses of our galaxy” Naming--Fullerene • The substance of the Fullerene was named after the American inventor, architects and philosophers Richard Buckminster Fuller (1895 till 1983). As an architect, R.B.Fuller designed the constructions which exist of 5-corners and 6corners, for example, the American pavilion to the Expo in '67 in Montréal(geodesic dome). • Fullerenes are similar in structure to graphite, which is composed of stacked graphene sheets of linked hexagonal rings; but they may also contain pentagonal (or sometimes heptagonal) rings. Buckminster Fuller Buckminster Fuller Richard Buckminster Fuller, c. 1917. Born July 12, 1895 Milton, Massachusetts, United States Died July 1, 1983 (aged 87) Los Angeles, United States Occupation Visionary, designer,architect, author, inventor Spouse Anne Fuller Children 2: Allegra Fuller Snyder and Alexandra who died in childhood Richard Buckminster Fuller, c. 1917 The Montreal Biosphère by Buckminster Fuller, 1967 After the IUPAC nomenclature the C60 has the following name: [29.29.0.0.2,14.03,12. 04,59.05,10.06,58. 07,55.08,53.09,21. 011,20.013,18.015,30. 016,28.017,25.019,24. 022,52. 023.50.026,49.027,47. 029,45.032,44.033,60. 034,57.035,43.036,56. 037,41.038,54.039,51. 040,48.042,46]hexaconta1,3,5(10),6,8,11,13(18), 14,16,19,21,23,25,27,29(45),30,32(44),33,35(43), 36,38(54),39(51),40(48),41,46,49,52,55,57,59-triacont IUPAC--International Union of Pure and Applied Chemistry FULLERENE MOLECULAR MODELS C56-C76 C56 (Td) Model C58 (C3v) C56 (Td) Rotating Animation C60(C2v) C76 (D2) C60(C2v) C72 (D6d) Different structure buckyballs C20 C26 C60 C70 Another fairly common fullerene is C70, but fullerenes with 72, 76, 84 and even up to 100 carbon atoms are commonly obtained. In mathematical terms, the structure of a fullerene is a trivalent convex polyhedron with pentagonal and hexagonal faces. In graph theory, the term fullerene refers to any 3-regular, planar graph with all faces of size 5 or 6 (including the external face). It follows from Euler's polyhedron formula, V − E + F = 2, (where V, E, F are the numbers of vertices, edges, and faces), that there are exactly 12 pentagons in a fullerene and V/2 − 10 hexagons. Fullerene Family Fig. 3.1: A schematic representation of the structures of graphite, diamond and fullerenes. While the two-dimensional sheets formed by hexagons are packed one over another in graphite, the diamond structure is three-dimensional. Only two fullerenes are shown. The smaller one is buckminsterfullerene, C60. structure. The double bonds are localized exocyclic to the pentagons giving [5] radialene character to the pentagons and cyclohexa-1,3,5-triene character to the hexagons. Synthesis(discover) of C60 Fig. 3.2: The experimental set-up used to discover C60. The graphite disk is evaporated with a Nd:YAG laser and the evaporated carbon plasma is cooled by a stream of helium coming from a pulsed valve. The clusters of carbon are produced in the integration cup and are expanded into vacuum. The ions are detected by time of flight mass spectrometry synthesis and purification of fullerenes Fig. 3.4: Schematic illustration of the processed involved in the synthesis and purification of fullerenes. Graphite rods are evaporated in an arc, under He atmosphere. The soot collected is extracted with toluene and subjected to chromatography. Properties & Applications •In April 2003, fullerenes were under study for potential medicinal use: binding specific antibiotics to the structure to target resistant bacteria and even target certain cancer cells •use of fullerenes as light-activated antimicrobial agents C60 in solution Fullerenes are stable, but not totally unreactive. Solubility Fullerenes are sparingly soluble in many solvents Solutions of pure buckminsterfullerene have a deep purple color. Solutions of C70 are a reddish brown. The higher fullerenes C76 to C84 have a variety of colors Properties & Applications Fig. 3.6: (Bottom) Mass spectrum of a laser evaporated C60 film showing coalescence of fullerenes. Mass peaks are seen at (C60)n (Ref.64). (Top) Collision of high energy ions on C60 results in the addition of C2s to C60. The mass spectrum here shows the addition of a number of such species (Ref.65). Combined figure originally published in, T. Pradeep, Current Science, 72 (1997) 124. Properties & Applications Hydrated Fullerene Hydrated fullerene C60HyFn is a stable, highly hydrophilic, supra-molecular complex consisting of С60 fullerene molecule enclosed into the first hydrated shell that contains 24 water molecules: C60@(H2O)24. C60HyFn water solution with a C60 concentration of 0.22 mg/mL Superconductivity that intercalation of alkali-metal atoms in solid C60 leads to metallic behavior. In 1991, it was revealed that potassium-doped C60 becomes superconducting at 18 K. This was the highest transition temperature for a molecular superconductor. Since then, superconductivity has been reported in fullerene doped with various other alkali metals Properties & Applications Fig. 3.8: Normalized DC electrical resistivity ρ(T)of a K3 C60 single crystal. The Tc observed is 19.8K. ρ0 is the resistivity at T=280K. Reprinted with permission from Xiang, et al. (Ref.95). Copyright (1992) AAAS. Summary and Reference 1 a interesting video: http://v.youku.com/v_show/id_XMjM4NDcwMzcy.html 2 Our course website: http://cc.usst.edu.cn/Able.Acc2.Web/Template/View.aspx?courseType=0&co urseId=458&topMenuId=49182&menuType=4&action=view&type=&name= 3 Quite useful website:( wikipedia website) http://en.wikipedia.org/wiki/Fullerene 4 Reference books: T.PRADEEP NANO –The essentials, understanding nanoscience and nanotechnology. Springer handbook of nanotechnology (Bhushan Editor) NanoArt Gallary “Ancient of Days” from classical art to quantum art THANK YOU!