Concentration Calculations: ppm, ppb, Molarity, Dilutions
advertisement

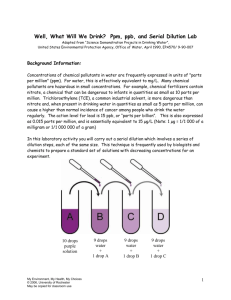
More Concentrations (ppm/ppb, molarity and dilutions) Learning Goals: I will be able define ppm and ppb units and apply knowledge of them to perform the necessary calculations to solve for very small concentrations I will be able to define what molar concentration (molarity) means, and apply it to solve for the molar concentration of a given solution, as well as unknowns in diluted solutions Very Small Concentrations (ppm and ppb) Very dilute solutions have concentrations much less than 1% (m/m) Use ppm (parts per million) or ppb (parts per billion) for these 6 ppm= mass of solute x 10 mass of solution 9 mass of solute x 10 ppb= mass of solution Example: ppb problem Cadmium is a highly toxic metal. The average level of cadmium in the blood of Canadians is about 0.35ppb. At this level, what mass of cadmium would be present in 1.5 kg of blood? (Convert units to grams first!) Example # 2: ppm problem Health Canada’s guideline for max mercury levels in fish is 0.5 ppm. When a 1.6kg salmon was tested, it was found to contain 0.6 mg of Hg. Would this salmon be safe to eat? (Convert units to grams first!) You Try: ppb/ppm problem House paint produced in Canada must contain less than 600 ppm of lead. What is the maximum mass of lead permitted in a can that contains 7.0 kg of paint? (Convert units to g!) Molar Concentrations Molar Concentrations Also known as Molarity:the number of moles of solute that can dissolve in 1 L of solution. Molar concentration (mol/L) = Amount of solute (mol) Volume of solution (L) c= n/v n c V Example #1: Calculating Molarity What is the concentration, in mol/L, of a solution formed by dissolving 28.0g of calcium chloride in enough water to make 225 mL of solution? (Find nCaCl2 first using M, then c) Example #2: Calculating Molarity How many grams of sodium nitrate would be needed to make 425 mL of 6.00 mol/L solution? (Find n first using n=cV, then m using m=nxM) You Try #1: Calculating Molarity What final solution volume would be required to prepare A 0.100 mol/L solution of AgNO3(aq) if 1.20 g of the solid salt will be used? (Find nAgNO3 first, then use V=n/c to find V) You Try #2: Calculating Molarity A solution of sodium chloride in water contains 14.0 g of NaCl dissolved in 250 mL of solution. What is the molarity of the solution? Preparing Solutions in a Lab We can prepare standard solutions (a solution of an accurate, known concentration) from stock solutions (a concentrated solution) by using a volumetric flask (glassware that is used to make a liquid solution with an accurate volume) Watch video on preparing standard solutions using volumetric flasks: http://www.youtube.com/watch?v=A2YyIo8vSCA Preparing Diluted Solutions If dilute solutions are prepared accurately, no mass will be lost, and therefore the amount of moles will stay the same. Using c=n/v, we can rearrange to get n=cV, and since n is not changing if we dilute a solution, the formula below arises: c1V1=c2V2 Where c1 = initial concentration of solution before dilution v1 = volume of solution before dilution c2 = final concentration of solution after dilution v2 = volume of solution after dilution Example #1: Dilution Calculations Your teacher has a stock solution of 12 mol/L HCl (aq). A class experiment requires 2.0L of 0.10 mol/L of hydrochloric acid. What volume of concentrated solution should be used to make the dilute solution for the experiment? You Try: Dilution Calculations What volume of 1.25 mol/L KI solution can you make with 125 mL of 3.00 mol/L potassium iodide solution? How did we do? Learning Goals: I will be able define ppm and ppb units and apply knowledge of them to perform the necessary calculations to solve for very small concentrations I will be able to define what molar concentration (molarity) means, and apply it to solve for the molar concentration of a given solution, as well as unknowns in diluted solutions