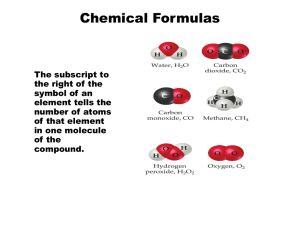
Chemistry 125: Lecture 28
November 8, 2010
Communicating Molecular Structure
in Diagrams and Words
Correct configuration is vital in drug molecules, like eribulin. It is important that chemists
agree on notation and nomenclature in order to communicate molecular constitution and
configuration. Clear notation also aids clarity of thought. The conventional 1891 Fischer
projection, which has been indispensable in understanding sugar configurations for over a
century, was invented in order to count stereoisomers. Ambiguity in diagrams or words has
led to multibillion dollar patent disputes involving popular pharmaceuticals. International
agreements provide descriptive, unambiguous, unique, systematic “IUPAC” names that are
reasonably convenient for most organic molecules of modest molecular weight. Also in 1891
Fischer devised the D,L “genealogical” scheme to describe relative configurations, but it can
be cumbersome or ambiguous.
Preliminary
For copyright
notice see final
page of this file
Halichondrin B: A Marine Natural Product with
Potent In Vitro and In Vivo Antitumor Activity
Discovery of E7389 Starting From the
Halichondrin B C.1-C.38 Macrolide
HO
O
HO
H
H
Active Fragment
of Halichondrin B
Structure-Activity
Relationship Study
Me
O
O
O
O
O O
Me
O
O
OMe
OH
H2N
H
O
O
H
19 “stereogenic centers”
O
O
H
SAR
Version
of Active Fragment
became Drug Candidate
E7389, now “Eribulin”
halichondrin B C.1-C.38 macrolide
H"Simplified"
H
O
O
H
O
H
O O
Me
O
H
O
E7389
"In Vitro and In Vivo Anticancer Activities of Synthetic Macrocyclic Ketone Analogues of Halichondrin B"
M.J. Towle, K.A. Salvato, J. Budrow, B.F. Wels, G. Kuznetsov, K.K. Aalfs, S. Welsh, W. Zheng, B.M.
Seletsky, M.H. Palme, G.J. Habgood, L.A. Singer, L.V. DiPietro, Y.Wang, J.J. Chen, D.A. Quincy, A.Davis
K. Yoshimatsu, Y. Kishi, M.J. Yu, and B.A. Littlefield, Cancer Res., February 1, 2001; 61(3): 1013-1021
219 > 500,000 configurational isomers!
ClinicalTrials.gov currently has 98,100 trials
with locations in 174 countries. (Nov. 3, 2010)
E7389 Phase III Nov 2008
(Nov.
2009)
(Nov. 3,
7, 2010)
2008)
Lipitor
A $10,000,000,000 Problem
in Stereochemical Notation
The world’s best selling drug ($10.86 billion in 2004)
Oct. 12, 2005
NEW YORK - Pfizer Inc. won a significant victory on
Wednesday when a British judge upheld a key patent covering
its blockbuster cholesterol drug Lipitor in the United Kingdom
but the medication still faces a similar yet more important case
in the United States. (Analogus decision 8/2/2006 in U.S. Court of Appeals)
Judge Nicholas Pumfrey upheld the patent covering
atorvastatin, Lipitor’s active ingredient, but ruled that another
patent was invalid. Indian pharmaceutical company Ranbaxy
Laboratories Ltd. had challenged both patents, and was joined
by Britain’s Arrow Generics Ltd. against the second patent that
was ruled invalid.
Pfizer said the decision upholding the exclusivity of the
patent covering atorvastatin until November 2011 was an
important victory for scientists.
What is Patented?
single enantiomer (Lipitor, drawn in figure)
…
or racemate (described
in text)
F
-
(CH2)2 OH O
Ca+2
O
H-N
i-Pr
Number of permutations
much greater than the
total number of protons,
neutrons, and electrons
in the Solar system!
Opinion of Sir Nicholas Pumfrey
In the '633 patent, it is absolutely clear from context
throughout that formula (I) is being used to denote a racemate.
In my judgment, every time the skilled person sees formula I or
formula X he will see it with eyes that tell him that in that
racemate, there is a single enantiomer that is the effective
compound, and that he can resolve the racemate using
conventional techniques to extract that enantiomer.
When one comes to claim 1, which echoes the purpose of the
invention with its conventional reference to pharmaceutically
Pfizer’s
latertopatent
acceptable salts, he will, in myThus
judgment,
continue
see the
formulae in this light.
on the single enantiomer
In my view, the claim
is an invalid repeat, and
covers the racemate and
patent protection will run
the individual enantiomers.
out three years earlier !
I
X
Constitutional Nomenclature Conventions
Geneva (1892)
IUPAC, International Union
of Pure & Applied Chemistry
http://www.chem.qmw.ac.uk/iupac/
http://acdlabs.com/iupac/nomenclature
Formal name should be:
• Clearly Descriptive
• Unambiguous
•
Composition, Constitution,
Configuration, Conformation
i.e. Stereochemistry
Unfortunately "Amide" means
One name one structure
both R2N and RCONH2
Eisai
graphics
Unique Indexing Alternatives: composition, computer
Research
One structure one name
• Manageable
Institute
(e.g. Quick, Easy, Short, Pronounceable)
Eribulin
Systematic Constitutional Nomenclature
bromo
Choose the “Main Chain”methyl
for Greek Root Name
• Longest
• Most Substituents
(to give simpler names)
Number the Chain Atoms
methyl
Br
6
7
3
2 1
5
4
chloro
Cl
2
ethyl
1
7-bromo-2-chloro-3-ethyl-6,7-dimethylnonane
?
Br
• Lowest number at
First Difference
Name Substituents
3
Cl
(1-chloroethyl)
octane
Br
Alphabetize (& Count)
Cl
http://acdlabs.com/iupac/nomenclature/93/r93_338.htm
WOW!
)
(
[
]
Thank God for pictures and computer graphics.
Some Useful Non-Systematic Names
Isopentane
Isobutane
X
X
Neopentane
X
X
Isopropyl
sec-Butyl
tert-Butyl
Neopentyl
Nomenclature Drill
available on course website.
Configurational Nomenclature
Phenomenological
d/l
+/-
Relative
D/L
Absolute
R/S
Configurational Nomenclature
m.p.
[a]D
140°C
0° (meso)
170°C
+13° (dextro +)
170°C
-13° (laevo -)
Phenomenological
Tartaric Acid Isomers
Genealogical (Fischer, 1891)
D-Tartaric Acid
Relative (by synthesis) to
HOCH2CH(OH)CHO
d-(+)-glyceraldehyde
Defined as “D”-glyceraldehyde (Fischer’s Guess)
Multi-Step
Synthesis
First of 210 Tables
Glyceraldehyde
D
D
Lactic Acid
(1978)
Paul D. Bartlett
age 24
Problem for Monday:
Consider HOMO/LUMO
alternatives during the
reaction of HI with
1,3-butanediol to give
4-iodo-2-butanol
>2000 Compounds
in the 210 Tables
24 years old
Absolute Configuration
J. M. Bijvoet
van't Hoff Laboratory, Univ. Utrecht
(1949-51)
Na Rb d-(L)-Tartrate
X-ray anomalous
dispersion
60 year old
Fischer Guess
for of our
“The question of nomenclature is beyond the scope
investigation... The problem of nomenclature (L)-Tartrate
now concerns
given configurations, and requires a notation which denotes
these configurations in an unambiguous and if possible selfexplanatory way.” (Bijvoet, 1951)
Naming Double Bond Configuration
Malic Acid
(HO2C)CH(OH).CH2. (CO2H)
D
(HO2C)HC=CH(CO2H)
Maleic & Fumaric Acids
Absolute nomenclature
is hard to generalize
(though relative is fine)
H3 C
cis
CH3
H
H
COOH
cis or trans?
H
H
COOH
D
HOOC
O
+ H2O
O
cis
COOH
O
(on this side of)
HOOC
H
trans
(across)
Double Bond Configuration
Assign groups at either end "priority"
by atomic number (or weight for isotopes)
at first difference
H
H3C
H
C
CH3
H
O
C
COOH
O
H
HO
O
HO
O
O
C
COOH
O
H3C
H
H
H
C
CH3
H
The names
trans and cis are "polluted"
previous usage.
(E)ntgegen
(Opposed)
(Z)by
usammen
(Together)
In Assigning Priority
Proceed One "Shell" at a Time
(respecting previous decisions)
O
H
O
C Win
O Cl is high
Tie
C
H
O
H
H
in priority,
but irrelevant;
H the decision Cl
is already made.
H3 C
O
Win
C
O
Tie
H
C
C
H
Cl
R. S.
Cahn
V.
C. K.
Ingold Prelog
R. Robinson
by permission J. D. Roberts
The 1950s "CIP" Priority Scheme is Conventional
R. B.Woodward
Robinson: "Hello Katchalsky. What are you doing here in Zurich?"
Prelog: "Excuse me, Sir Robert, I am only Prelog, and I live here."
Robinson: "You know, Prelog, your and Ingold's configurational
notation is all wrong."
Prelog: "Sir Robert, it can't be wrong. It is just a convention.
You either accept it or not."
Robinson: "Well then, if it is not wrong, it is absolutely unnecessary."
from V. Prelog, My 132 semesters of chemistry studies (1981)
http://www.bl.uk/onlinegallery/themes/euromanuscripts/lindisfarne.html
Exercise for
Monday:
Eadfrith’s
Error
(Click here & create your
very own chiral conventions)
D
H
HO
left
turn
1
CIP (R/S) Nomenclature
CH3 for Stereogenic Centers HO
2
2
CH3
2
D3
HO
H4
(S)inister (left)
H
HO
OH
3
H
D
right
turn
CH3
COOH
4
CH3
1
H
1
HO
H4
D3
(R)ectus (right)
COOH
H
(2R,3R)2,3-dihydroxy
butanedioic
acid
H
Bloomer Gate
Organic
End of Lecture 28
Nov. 8, 2010
Copyright © J. M. McBride 2009,2010. Some rights reserved. Except for cited third-party materials, and those used by
visiting speakers, all content is licensed under a Creative Commons License (Attribution-NonCommercial-ShareAlike 3.0).
Use of this content constitutes your acceptance of the noted license and the terms and conditions of use.
Materials from Wikimedia Commons are denoted by the symbol
.
Third party materials may be subject to additional intellectual property notices, information, or restrictions.
The following attribution may be used when reusing material that is not identified as third-party content:
J. M. McBride, Chem 125. License: Creative Commons BY-NC-SA 3.0