Chapter 15
advertisement

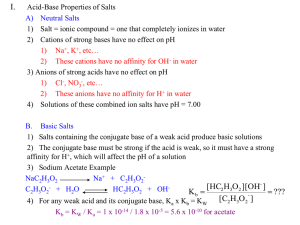
Chapter 15 Applications of Aqueous Equilibria 15.1 Neutralization Reaction General Formula Acid + Base Water + Salt Four types: Strong acid – strong base Weak acid – strong base Strong acid – Weak base Weak acid-Weak base To what extend does a neutralization Reaction go to completion? Strong Acid-Strong Base If we mix equal numbers moles of HCl(aq) and NaOH(aq), the [H3O+] and [-OH] remaining in solution after neutralization will be same as those in pure water HCl(aq) + NaOH(aq) H2O(l) + Assuming complete dissociation: (net ionic equation) H3O1+(aq) + OH1-(aq) [H3O+] = [-OH] = 1.0 x 10-7M Reaction proceeds far to the right pH = 7.00 Kn = 1.0 x 1014 2H2O(l) NaCl(aq) Weak Acid-Strong Base Because a weak acid HA is largely undissociated, the net equation for the neutralization reaction of a weak acid with a strong base involves proton transfer from HA to the strong base, -OH CH3CO2H(aq) + NaOH(aq) (net ionic equation) CH3CO2H(aq) + OH1-(aq) H2O(l) + NaCH3CO2(aq) H2O(l) + CH3CO21-(aq) Neutralization of any weak acid by a strong base goes 100% to completion -OH has a great infinity for protons pH >7.00 Kn > 107 Strong Acid-Weak Base A strong acid HA is completely dissociated into H3O+ and A- ions, and its neutralization reaction with a weak base involves proton transfer from H3O+ to the weak base B: HCl(aq) (net ionic equation) + NH3(aq) H3O1+(aq) + NH3 (aq) NH4Cl(aq) H2O(l) + NH41+(aq) Neutralization of any weak base by a strong acid goes 100% to completion H3O+ has a great infinity for protons pH < 7.00 Kn > 107 Weak Acid-Weak Base CH3CO2H(aq) + NH3(aq) NH4CH3CO2(aq) (net ionic equation) CH3CO2H(aq) + NH3(aq) NH41+(aq) + CH3CO21-(aq) After neutralization: pH = ? Weak acid-weak base neutralization has less tendency to proceed to completion than strong acids and strong bases. The Common-Ion Effect Common-Ion Effect: The shift in the position of an equilibrium on addition of a substance that provides an ion in common with one of the ions already involved in the equilibrium CH3CO2H(aq) + H2O(l) H3O+(aq) + CH3CO2–(aq) What are the ions present in the solution? 15.2 The Common-Ion Effect The pH of 0.10 M acetic acid is 2.89. Calculate the pH of a solution that is prepared by dissolved 0.10 mol of acetic acid and 0.10 mol sodium acetate in enough water to make 1.00 L of solution. Ka = 1.8 x 10-5 Step 1: Identify the mixture compositions Step 2: Consider all possible reactions Step 3: Identify the principle reaction Step 4: Calculate new pH Step 5 : Check your answer The Common-Ion Effect Le Châtelier’s Principle CH3CO2H(aq) + H2O(l) The addition of acetate ion to a solution of acetic acid suppresses the dissociation of the acid. The equilibrium shifts to the left. H3O+(aq) + CH3CO2–(aq) Example In 0.15 M NH3, the pH is 11.21 and the percent dissociation is 1.1%. Calculate the concentrations of NH3, pH and percent dissociation of ammonia in a solution that is 0.15M NH3 and 0.45 M NH4Cl Kb = 1.8 x 10-5 15.3 Buffer Solutions Buffer Solution: A solution which contains a weak acid and its conjugate base and resists drastic changes in pH. Weak acid + Conjugate base For Example: CH3CO2H + CH3CO21HF + F1NH41+ + NH3 H2PO41- + HPO42- Buffer Solutions Add a small amount of base (-OH) to a buffer solution ◦ Acid component of solution neutralizes the added base Add a small amount of acid (H3O+) to a buffer solution ◦ Base component of solution neutralizes the added acid The addition of –OH or H3O+ to a buffer solution will change the pH of the solution, but not as drastically as the addition of –OH or H3O+ to a non-buffered solution Buffer Solutions HA(aq) + H2O(l) H3O1+(aq) + A1-(aq) Weak acid Conjugate base (M+A-) Addition of OH1- to a buffer: HA(aq) + OH1-(aq) 100% H2O(l) + A1-(aq) Addition of H3O1+ to a buffer: A1-(aq) + H3O1+(aq) 100% H2O(l) + HA(aq) Buffer Solutions Buffer Solutions Example pH of human blood (pH = 7.4) controlled by conjugated acid-base pairs (H2CO3/HCO3-). Write a neutralization equation for the following effects ◦ With addition of HCl ◦ With addition of NaOH Example Calculate the pH of 0.100L of a buffer solution that is 0.25M in HF and 0.50 M in NaF. ◦ What is the change in pH on addition of 0.002 mol HCl ◦ What is the change in pH on addition of 0.010 moles KOH ◦ Calculate the pH after addition of 0.080 moles HBr • Assume the volume remains constant • Ka = 3.5 x 10-4 Example Calculate the pH of the buffer that results from mixing 60.0mL of 0.250M HCHO2 and 15.0 mL of 0.500M NaCHO2 Ka = 1.7 x 10-4 ◦ Calculate the pH after addition of 10.0 mL of 0.150 MHBr. Assume volume is additive Buffer Capacity A measure of amount of acid or base that the solution can absorb without a significant change in pH. Depends on how many moles of weak acid and conjugated base are present. For an equal volume of solution: the more concentrated the solution, the greater buffer capacity For an equal concentration: the greater the volume, the greater the buffer capacity Example The following pictures represent solutions that contain a weak acid HA and/ or its sodium salt NaA. (Na+ ions and solvent water molecules have been omitted for clarity Which of the solutions are buffer solution? Which solution has the greatest buffer capacity? 15.4 The Henderson-Hasselbalch Equation Weak acid Conjugate base Acid(aq) + H2O(l) Ka = [H3O1+][Base] [H3O1+] = Ka [Acid] pH = pKa + log H3O1+(aq) + Base(aq) [Base] [Acid] [Acid] [Base] Examples Calculate the pH of a buffer solution that is 0.50 M in benzoic acid (HC7H5O2) and 0.150 M in sodium benzoate (NaC7H5O2). Ka = 6.5 x 10-5 How would you prepare a NaHCO3-Na2CO3 buffer solution that has pH = 10.40 Ka2 = 4.7 x 10-11