19.1
Acid-Base Theories
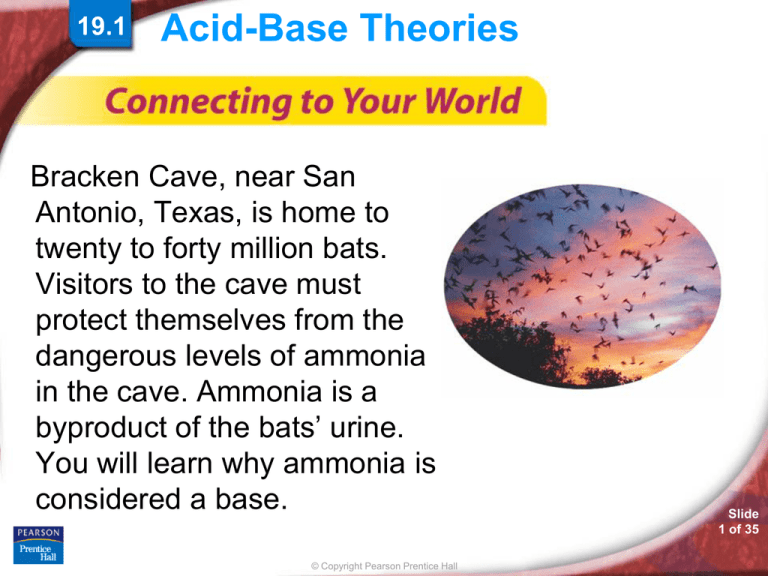
Bracken Cave, near San
Antonio, Texas, is home to
twenty to forty million bats.
Visitors to the cave must
protect themselves from the
dangerous levels of ammonia
in the cave. Ammonia is a
byproduct of the bats’ urine.
You will learn why ammonia is
considered a base.
© Copyright Pearson Prentice Hall
Slide
1 of 35
19.1
Acid-Base Theories
> Properties of Acids and Bases
Properties of Acids and Bases
What are the properties of acids and
bases?
Slide
2 of 35
© Copyright Pearson Prentice Hall
19.1
Acid-Base Theories
> Properties of Acids and Bases
Acids
Acids taste sour, will change the color
of an acid-base indicator, and can be
strong or weak electrolytes in
aqueous solution.
Slide
3 of 35
© Copyright Pearson Prentice Hall
19.1
Acid-Base Theories
> Properties of Acids and Bases
Citrus fruits contain citric acid. Tea contains
tannic acid.
Slide
4 of 35
© Copyright Pearson Prentice Hall
19.1
Acid-Base Theories
> Properties of Acids and Bases
Bases
Bases taste bitter, feel slippery, will
change the color of an acid-base
indicator, and can be strong or weak
electrolytes in aqueous solution.
Slide
5 of 35
© Copyright Pearson Prentice Hall
19.1
Acid-Base Theories
> Properties of Acids and Bases
Antacids use bases to neutralize excess
stomach acid. The base calcium hydroxide is a
component of mortar.
Slide
6 of 35
© Copyright Pearson Prentice Hall
19.1
Acid-Base Theories
> Arrhenius Acids and Bases
Arrhenius Acids and Bases
How did Arrhenius define an acid and
a base?
Slide
7 of 35
© Copyright Pearson Prentice Hall
19.1
Acid-Base Theories
> Arrhenius Acids and Bases
Arrhenius said that acids are hydrogencontaining compounds that ionize to
yield hydrogen ions (H+) in aqueous
solution. He also said that bases are
compounds that ionize to yield
hydroxide ions (OH–) in aqueous
solution.
Slide
8 of 35
© Copyright Pearson Prentice Hall
19.1
Acid-Base Theories
> Arrhenius Acids and Bases
Hydrochloric Acid
Slide
9 of 35
© Copyright Pearson Prentice Hall
19.1
Acid-Base Theories
> Arrhenius Acids and Bases
Arrhenius Acids
• Acids that contain one ionizable hydrogen,
such as nitric acid (HNO3), are called
monoprotic acids.
• Acids that contain two ionizable hydrogens,
such as sulfuric acid (H2SO4), are called
diprotic acids.
• Acids that contain three ionizable hydrogens,
such as phosphoric acid (H3PO4) are called
triprotic acids.
© Copyright Pearson Prentice Hall
Slide
10 of 35
19.1
Acid-Base Theories
> Arrhenius Acids and Bases
Slide
11 of 35
© Copyright Pearson Prentice Hall
19.1
Acid-Base Theories
> Arrhenius Acids and Bases
Arrhenius Bases
Hydroxide ions are one of the products of the
dissolution of an alkali metal in water.
Slide
12 of 35
© Copyright Pearson Prentice Hall
19.1
Acid-Base Theories
> Arrhenius Acids and Bases
Slide
13 of 35
© Copyright Pearson Prentice Hall
19.1
Acid-Base Theories
> Brønsted-Lowry Acids and Bases
Brønsted-Lowry Acids and Bases
What distinguishes an acid from a base
in the Brønsted-Lowry theory?
Slide
14 of 35
© Copyright Pearson Prentice Hall
19.1
Acid-Base Theories
> Brønsted-Lowry Acids and Bases
The Brønsted-Lowry theory defines an
acid as a hydrogen-ion donor, and a
base as a hydrogen-ion acceptor.
Slide
15 of 35
© Copyright Pearson Prentice Hall
19.1
Acid-Base Theories
> Brønsted-Lowry Acids and Bases
Why Ammonia is a Base
Slide
16 of 35
© Copyright Pearson Prentice Hall
19.1
Acid-Base Theories
> Brønsted-Lowry Acids and Bases
Conjugate Acids and Bases
• A conjugate acid is the particle formed when
a base gains a hydrogen ion.
• A conjugate base is the particle that remains
when an acid has donated a hydrogen ion.
Slide
17 of 35
© Copyright Pearson Prentice Hall
19.1
Acid-Base Theories
> Brønsted-Lowry Acids and Bases
• A conjugate acid-base pair consists of two
substances related by the loss or gain of a
single hydrogen ion.
• A substance that can act as both an acid and
a base is said to be amphoteric.
Slide
18 of 35
© Copyright Pearson Prentice Hall
19.1
Acid-Base Theories
> Brønsted-Lowry Acids and Bases
Slide
19 of 35
© Copyright Pearson Prentice Hall
19.1
Acid-Base Theories
> Brønsted-Lowry Acids and Bases
A water molecule that gains a hydrogen ion
becomes a positively charged hydronium ion
(H3O+).
Slide
20 of 35
© Copyright Pearson Prentice Hall
19.1
Acid-Base Theories
> Lewis Acids and Bases
Lewis Acids and Bases
How did Lewis define an acid and a
base?
Slide
21 of 35
© Copyright Pearson Prentice Hall
19.1
Acid-Base Theories
> Lewis Acids and Bases
Lewis proposed that an acid accepts a
pair of electrons during a reaction, while
a base donates a pair of electrons.
Slide
22 of 35
© Copyright Pearson Prentice Hall
19.1
Acid-Base Theories
> Lewis Acids and Bases
• A Lewis acid is a substance that can accept
a pair of electrons to form a covalent bond.
• A Lewis base is a substance that can donate
a pair of electrons to form a covalent bond.
Slide
23 of 35
© Copyright Pearson Prentice Hall
Acid-Base Theories
> Lewis Acids and Bases
Animation 25
Compare the three important definitions of
acids and bases.
Slide
24 of 35
© Copyright Pearson Prentice Hall
19.1
Acid-Base Theories
> Lewis Acids and Bases
Slide
25 of 35
© Copyright Pearson Prentice Hall
Acid-Base Theories
>
Slide
26 of 35
© Copyright Pearson Prentice Hall
Acid-Base Theories
>
Slide
27 of 35
© Copyright Pearson Prentice Hall
Acid-Base Theories
>
Slide
28 of 35
© Copyright Pearson Prentice Hall
Practice Problems for Conceptual Problem 19.1
Problem Solving 19.1
Solve Problem 1 with the help of an
interactive guided tutorial.
Slide
29 of 35
© Copyright Pearson Prentice Hall
19.1 Section Quiz.
1. Which of the following is NOT a characteristic
of acids?
a. taste sour
b. are electrolytes
c. feel slippery
d. affect the color of indicators
Slide
30 of 35
© Copyright Pearson Prentice Hall
19.1 Section Quiz.
2. Which compound is most likely to act as an
Arrhenius acid?
a. H2O
b. NH3.
c. NaOH.
d. H2SO4.
Slide
31 of 35
© Copyright Pearson Prentice Hall
19.1 Section Quiz.
3. A Lewis acid is any substance that can accept
a. a hydronium ion.
b. a proton.
c. hydrogen.
d. a pair of electrons.
Slide
32 of 35
© Copyright Pearson Prentice Hall
19.2
Hydrogen Ions and Acidity
To test a diagnosis of
diabetic coma, a doctor
orders several tests,
including the acidity of
the patient’s blood.
Results from this test
will be expressed in
units of pH. You will
learn how the pH scale
is used to indicate the
acidity of a solution and
© Copyright Pearson Prentice Hall
Slide
33 of 35
19.2
Hydrogen
Ions from Water
Hydrogen Ions from Water
a. The reaction in which water molecules
produce ions is called the self-ionization
of water.
Slide
34 of 35
© Copyright Pearson Prentice Hall
19.2
Hydrogen
Ions from Water
a. In the self-ionization of water, a proton
(hydrogen ion) transfers from one water
molecule to another water molecule.
Slide
35 of 35
© Copyright Pearson Prentice Hall
19.2
Ion Product
Constant for Water
Ion Product Constant for Water
How are [H+] and [OH-] related in an aqueous
solution?
Slide
36 of 35
© Copyright Pearson Prentice Hall
19.2
Ion Product
Constant for Water
For aqueous solutions, the product of
the hydrogen-ion concentration
and the hydroxide-ion
concentration equals 1.0 10-14.
Any aqueous solution in which [H+] and
[OH-] are equal is described as a
neutral solution.
Slide
37 of 35
© Copyright Pearson Prentice Hall
19.2
Ion Product
Constant for Water
a. The product of the concentrations of the
hydrogen ions and hydroxide ions in water
is called the ion-product constant for
water (Kw).
Slide
38 of 35
© Copyright Pearson Prentice Hall
19.2
Ion Product
Constant for Water
a. An acidic solution is one in which [H+] is
greater than [OH-].
Slide
39 of 35
© Copyright Pearson Prentice Hall
19.2
Ion Product
Constant for Water
a. Unrefined hydrochloric acid, commonly
called muriatic acid, is used to clean stone
buildings and swimming pools.
Slide
40 of 35
© Copyright Pearson Prentice Hall
19.2
Ion Product
Constant for Water
a. A basic solution is one in which [H+] is
less than [OH]. Basic solutions are also
known as alkaline solutions.
Slide
41 of 35
© Copyright Pearson Prentice Hall
19.2
Ion Product
Constant for Water
a. Sodium hydroxide, or lye, is commonly
used as a drain cleaner.
Slide
42 of 35
© Copyright Pearson Prentice Hall
19.1
Slide
43 of 35
© Copyright Pearson Prentice Hall
19.1
Slide
44 of 35
© Copyright Pearson Prentice Hall
19.1
Slide
45 of 35
© Copyright Pearson Prentice Hall
19.1
Slide
46 of 35
© Copyright Pearson Prentice Hall
for Sample Problem 19.1
Slide
47 of 35
© Copyright Pearson Prentice Hall
The 19.2
pH Concept
The pH Concept
How is the hydrogen-ion concentration used to
classify a solution as neutral, acidic, or basic?
Slide
48 of 35
© Copyright Pearson Prentice Hall
The 19.2
pH Concept
a. The pH of a solution is the negative
logarithm of the hydrogen-ion
concentration.
Slide
49 of 35
© Copyright Pearson Prentice Hall
The 19.2
pH Concept
Calculating pH
Slide
50 of 35
© Copyright Pearson Prentice Hall
The 19.2
pH Concept
a. A solution in which [H+] is greater than 1
10–7 M has a pH less than 7.0 and is
acidic. The pH of pure water or a neutral
aqueous solution is 7.0. A solution with a
pH greater than 7 is basic and has a [H+] of
less than 1 10–7 M.
Slide
51 of 35
© Copyright Pearson Prentice Hall
The 19.2
pH Concept
Slide
52 of 35
© Copyright Pearson Prentice Hall
The 19.2
pH Concept
Slide
53 of 35
© Copyright Pearson Prentice Hall
The 19.2
pH Concept
Calculating pOH
Slide
54 of 35
© Copyright Pearson Prentice Hall
The 19.2
pH Concept
pH and Significant Figures
Slide
55 of 35
© Copyright Pearson Prentice Hall
19.2
Slide
56 of 35
© Copyright Pearson Prentice Hall
19.2
Slide
57 of 35
© Copyright Pearson Prentice Hall
19.2
Slide
58 of 35
© Copyright Pearson Prentice Hall
19.2
Slide
59 of 35
© Copyright Pearson Prentice Hall
for Sample Problem 19.2
Slide
60 of 35
© Copyright Pearson Prentice Hall
19.3
Slide
61 of 35
© Copyright Pearson Prentice Hall
19.3
Slide
62 of 35
© Copyright Pearson Prentice Hall
19.3
Slide
63 of 35
© Copyright Pearson Prentice Hall
19.3
Slide
64 of 35
© Copyright Pearson Prentice Hall
for Sample Problem 19.3
Slide
65 of 35
© Copyright Pearson Prentice Hall
19.2
Measuring
pH
Measuring pH
What is the most important characteristic of an
acid-base indicator?
Slide
66 of 35
© Copyright Pearson Prentice Hall
19.2
Measuring
pH
An indicator is a valuable tool for measuring pH
because its acid form and base form have
different colors in solution.
Slide
67 of 35
© Copyright Pearson Prentice Hall
19.2
Measurin
g pH
a. Phenolphthalein changes from colorless to
pink at pH 7–9.
Slide
68 of 35
© Copyright Pearson Prentice Hall
19.4
Slide
69 of 35
© Copyright Pearson Prentice Hall
19.4
Slide
70 of 35
© Copyright Pearson Prentice Hall
19.4
Slide
71 of 35
© Copyright Pearson Prentice Hall
19.4
Slide
72 of 35
© Copyright Pearson Prentice Hall
for Sample Problem 19.4
Slide
73 of 35
© Copyright Pearson Prentice Hall
19.2
Measuring
pH
Acid-Base Indicators
Slide
74 of 35
© Copyright Pearson Prentice Hall
19.2
Measuring
pH
Slide
75 of 35
© Copyright Pearson Prentice Hall
19.2
Measuring
pH
a. Universal Indicators
Slide
76 of 35
© Copyright Pearson Prentice Hall
19.2 Section Quiz.
1. If the [OH-] in a solution is 7.65 10-3M, what
is the [H+] of this solution?
a. 7.65 10-17M
b. 1.31 10-12M
c. 2.12M
d. 11.88M
Slide
77 of 35
© Copyright Pearson Prentice Hall
19.2 Section Quiz.
2. The [OH-] for four solutions is given below.
Which one of the solution is basic?
a. 1.0 x 10-6M
b. 1.0 x 10-8M
c. 1.0 x 10-7M
d. 1.0 x 10-14M
Slide
78 of 35
© Copyright Pearson Prentice Hall
19.2 Section Quiz.
3. What is the pH of a solution with a hydrogenion concentration of 8.5 x 10-2M?
a. 12.93
b. 8.50
c. 5.50
d. 1.07
Slide
79 of 35
© Copyright Pearson Prentice Hall
19.3
Strengths of Acids and Bases
Lemons and grapefruits
have a sour taste
because they contain
citric acid. Sulfuric acid
is a widely used
industrial chemical that
can quickly cause
severe burns if it comes
into contact with skin.
You will learn why some
acids are weak and
© Copyright Pearson Prentice Hall
Slide
80 of 35
19.3
Strong and Weak
Acids and Bases
Strong and Weak Acids and Bases
How does the value of an acid dissociation
constant relate to the strength of an acid?
Slide
81 of 35
© Copyright Pearson Prentice Hall
19.3
Strong
and Weak Acids and Bases
a. An acid dissociation constant (Ka) is the
ratio of the concentration of the dissociated
(or ionized) form of an acid to the
concentration of the undissociated
(nonionized) form.
Slide
82 of 35
© Copyright Pearson Prentice Hall
19.3
Strong
and Weak Acids and Bases
Weak acids have small Ka values. The stronger
an acid is, the larger is its Ka value.
Slide
83 of 35
© Copyright Pearson Prentice Hall
19.3
Strong
and Weak Acids and Bases
a. Strong acids are completely ionized in
aqueous solution.
a. Weak acids ionize only slightly in aqueous
solution.
Slide
84 of 35
© Copyright Pearson Prentice Hall
19.3
Strong
and Weak Acids and Bases
a. In general, the base dissociation
constant (Kb) is the ratio of the
concentration of the conjugate acid times
the concentration of the hydroxide ion to
the concentration of the base.
Slide
85 of 35
© Copyright Pearson Prentice Hall
19.3
Strong
and Weak Acids and Bases
a. Strong bases dissociate completely into
metal ions and hydroxide ions in aqueous
solution.
b. Weak bases react with water to form the
hydroxide ion and the conjugate acid of the
base.
Slide
86 of 35
© Copyright Pearson Prentice Hall
19.3
Strong
and Weak Acids and Bases
Slide
87 of 35
© Copyright Pearson Prentice Hall
19.3
Calculating
Dissociation Constants
Calculating Dissociation Constants
How can you calculate an acid dissociation
constant (Ka) of a weak acid?
Slide
88 of 35
© Copyright Pearson Prentice Hall
19.3
Calculating
Dissociation
Constants
To find the Ka of a weak acid or the Kb of a weak
base, substitute the measured concentrations
of all the substances present at equilibrium
into the expression for Ka or Kb.
Slide
89 of 35
© Copyright Pearson Prentice Hall
Calculating
Dissociation
Constants
19.3
Acid Dissociation Constant
a. The dissociation constant, Ka, of ethanoic
acid is calculated from the equilibrium
concentrations of all of the molecules and
ions in the solution.
Slide
90 of 35
© Copyright Pearson Prentice Hall
19.3
Calculating
Dissociation
Constants
Slide
91 of 35
© Copyright Pearson Prentice Hall
19.3
Calculating
Dissociation
Constants
Slide
92 of 35
© Copyright Pearson Prentice Hall
Calculating
Dissociation
Constants
19.3
Base Dissociation Constant
a. The dissociation constant, Kb, of ammonia
is calculated from the equilibrium
concentrations of all of the molecules and
ions in the solution.
Slide
93 of 35
© Copyright Pearson Prentice Hall
Calculating
Dissociation
Constants
19.3
Concentration and Strength
Slide
94 of 35
© Copyright Pearson Prentice Hall
19.5
Slide
95 of 35
© Copyright Pearson Prentice Hall
19.5
Slide
96 of 35
© Copyright Pearson Prentice Hall
19.5
Slide
97 of 35
© Copyright Pearson Prentice Hall
19.5
Slide
98 of 35
© Copyright Pearson Prentice Hall
for Sample Problem 19.5
Problem Solving 19.23
Solve Problem 23 with the help
of an interactive guided
tutorial.
© Copyright Pearson Prentice Hall
Slide
99 of 35
19.3 Section Quiz.
1. H2S is considered to be a weak acid because it
a. is insoluble in water.
b. ionizes only slightly.
c. is completely ionized.
d. is dilute.
Slide
100 of 35
© Copyright Pearson Prentice Hall
19.3 Section Quiz.
2. Calcium hydroxide, Ca(OH)2, is a strong base
because it
a. has a large Kb.
b. has a small Kb.
c. forms concentrated solutions.
d. is highly soluble in water.
Slide
101 of 35
© Copyright Pearson Prentice Hall
19.3 Section Quiz.
3. If the [H+] of a 0.205M solution of phenol
(C6H5OH) at 25ºC is 2.340 10-6, what is the
Ka for phenol? Phenol is monoprotic.
a. Ka = 2.67 x 10-11
b. Ka = 1.14 x 10-5
c.
Ka = 5.48 x 10-12
d. Ka = 1.53 x 10-3
Slide
102 of 35
© Copyright Pearson Prentice Hall
19.3 Section Quiz.
4. The Ka of three acids is given below.
(1) 5.1 10–3
(2) 4.8 10–11
(3) 6.3 10–5
Put the acids in order from the strongest acid to the
weakest acid.
a. 1, 3, 2
b. 2, 3, 1
c. 3, 1, 2
Slide
103 of 35
d. 2, 1, 3
© Copyright Pearson Prentice Hall
19.3 Section Quiz.
5. The Kb of four bases is given below.
(1) 7.41 x 10-5
(2) 1.78 x 10-5
(3) 4.27 x 10-4
(4) 4.79 x 10-4
Put the bases in order from the strongest base to the weakest base.
a.
2, 3, 4, 1
b.
2, 1, 3, 4
c.
4, 3, 1, 2
d.
1, 4, 3, 2
Slide
104 of 35
© Copyright Pearson Prentice Hall
19.4
Neutralization Reactions
Excess hydrochloric acid in the stomach can
cause heartburn and a feeling of nausea.
Antacids neutralize the stomach acid and
relieve the pain of acid indigestion. You will
learn what a neutralization reaction is.
Slide
105 of 35
© Copyright Pearson Prentice Hall
19.4
Acid-Base
Reactions
Acid-Base Reactions
What are the products of the reaction of an acid
with a base?
Slide
106 of 35
© Copyright Pearson Prentice Hall
19.4
Acid-Base
Reactions
a. In general, the reaction of an acid with a
base produces water and one of a class of
compounds called salts.
Slide
107 of 35
© Copyright Pearson Prentice Hall
19.4
Acid-Base
Reactions
a. Reactions in which an acid and a base
react in an aqueous solution to produce a
salt and water are generally called
neutralization reactions.
Slide
108 of 35
© Copyright Pearson Prentice Hall
19.4
Acid-Base
Reactions
Slide
109 of 35
© Copyright Pearson Prentice Hall
19.4
Titration
Titration
What is the endpoint of a titration?
Slide
110 of 35
© Copyright Pearson Prentice Hall
19.4
Titration
a. The process of adding a known amount of
solution of known concentration to
determine the concentration of another
solution is called titration.
The point of neutralization is the end point
of the titration.
Slide
111 of 35
© Copyright Pearson Prentice Hall
19.4
Titration
a. When an acid and base are mixed, the
equivalence point is when the number of
moles of hydrogen ions equals the number
of moles of hydroxide ions.
Slide
112 of 35
© Copyright Pearson Prentice Hall
19.6
Slide
113 of 35
© Copyright Pearson Prentice Hall
19.6
Slide
114 of 35
© Copyright Pearson Prentice Hall
19.6
Slide
115 of 35
© Copyright Pearson Prentice Hall
19.6
Slide
116 of 35
© Copyright Pearson Prentice Hall
for Sample Problem 19.6
Slide
117 of 35
© Copyright Pearson Prentice Hall
19.4
Titration
a. The solution of known concentration is
called the standard solution.
Indicators are often used to determine
when enough of the standard solution
has been added to neutralize the acid or
base.
The point at which the indicator changes
color is the end point of the titration.
Slide
118 of 35
© Copyright Pearson Prentice Hall
19.4
Titration
Acid solution
with indicator
Added base is
measured with a
buret.
© Copyright Pearson Prentice Hall
Color change
shows
neutralization.
Slide
119 of 35
Titration
Simulation 26
Simulate the titration of several acids and bases
and observe patterns in the pH at
equivalence.
Slide
120 of 35
© Copyright Pearson Prentice Hall
19.4
Titration
Slide
121 of 35
© Copyright Pearson Prentice Hall
19.7
Slide
122 of 35
© Copyright Pearson Prentice Hall
19.7
Slide
123 of 35
© Copyright Pearson Prentice Hall
19.7
Slide
124 of 35
© Copyright Pearson Prentice Hall
19.7
Slide
125 of 35
© Copyright Pearson Prentice Hall
for Sample Problem 19.7
Problem Solving 19.33
Solve Problem 33 with the help of
an interactive guided tutorial.
Slide
126 of 35
© Copyright Pearson Prentice Hall
19.4 Section Quiz
1. When a neutralization takes place, one of the
products is always
a. carbon dioxide.
b. a salt.
c. sodium chloride.
d. a precipitate.
Slide
127 of 35
© Copyright Pearson Prentice Hall
19.4 Section Quiz
2. In a titration, 45.0 mL of KOH is neutralized by
75.0 mL of 0.30M HBr. What is the
concentration of the KOH solution?
a. 0.18M
b. 0.60M
c. 0.25M
d. 0.50M
Slide
128 of 35
© Copyright Pearson Prentice Hall
19.4 Section Quiz
3. How many moles of HCl are required to
neutralize an aqueous solution of 2.0 mol
Ca(OH)2?
a. 0.5 mol
b. 1.0 mol
c. 2.0 mol
d. 4.0 mol
Slide
129 of 35
© Copyright Pearson Prentice Hall
19.4 Section Quiz
4. In which of the following neutralization
titrations of 1-molar solutions of H2SO4 and
NaOH will the equivalence point be reached
at the very end of the additions?
H2SO4(aq) + 2NaOH(aq) Na2SO4(aq) +
2H2O(aq)
a. 200 mL of H2SO4 is slowly added to 100
mL of NaOH
b. 200 mL of H2SO4 is slowly added to 200
mL of NaOH
© Copyright Pearson Prentice Hall
Slide
130 of 35
19.5
Salts in Solution
The chemical processes inside a living cell are
very sensitive to pH. Human blood is
normally maintained at a pH very close to
7.4. You will learn about chemical processes
that ensure that the pH of blood is kept near
7.4.
Slide
131 of 35
© Copyright Pearson Prentice Hall
19.5
Salt Hydrolysis
Salt Hydrolysis
When is the solution of a salt acidic or basic?
Slide
132 of 35
© Copyright Pearson Prentice Hall
19.5
Salt Hydrolysis
In general, salts that produce acidic solutions
contain positive ions that release protons to
water. Salts that produce basic solutions
contain negative ions that attract protons from
water.
Slide
133 of 35
© Copyright Pearson Prentice Hall
19.5
Salt Hydrolysis
a. In salt hydrolysis, the cations or anions of
a dissociated salt remove hydrogen ions
from or donate hydrogen ions to water.
Slide
134 of 35
© Copyright Pearson Prentice Hall
19.5
Salt Hydrolysis
Slide
135 of 35
© Copyright Pearson Prentice Hall
19.5
Salt Hydrolysis
Slide
136 of 35
© Copyright Pearson Prentice Hall
19.5
Salt Hydrolysis
a. To determine whether a salt solution is
acidic or basic, remember the following
rules:
Slide
137 of 35
© Copyright Pearson Prentice Hall
19.5
Salt Hydrolysis
a. Vapors of the strong acid HCl(aq) and the
weak base NH3(aq) combine to form the
acidic white salt ammonium chloride
(NH4Cl).
Slide
138 of 35
© Copyright Pearson Prentice Hall
19.5
Salt Hydrolysis
Slide
139 of 35
© Copyright Pearson Prentice Hall
19.5
Salt Hydrolysis
a. Universal indicator solution has been
added to each of these 0.10M aqueous
salt solutions.
NH4Cl
pH 5.3
NaCl
pH 7
CH3COONa
pH 5.3
Slide
140 of 35
© Copyright Pearson Prentice Hall
19.5
Buffers
Buffers
What are the components of a buffer?
Slide
141 of 35
© Copyright Pearson Prentice Hall
19.5
Buffers
a. A buffer is a solution of a weak acid and
one of its salts, or a solution of a weak
base and one of its salts.
a. The pH of a buffer remains
relatively constant when small
amounts of acid or base are added.
b. The buffer capacity is the amount
of acid or base that can be added to
a buffer solution before a significant
change in pH occurs.
Slide
142 of 35
© Copyright Pearson Prentice Hall
19.5
Buffers
a. Buffer of Ethanoic Acid and Sodium
Ethanoate
Adding H+ produces additional ethanoic acid.
Adding OH- produces additional ethanoate
ions.
The pH changes very little.
Slide
143 of 35
© Copyright Pearson Prentice Hall
19.5
Buffers
Slide
144 of 35
© Copyright Pearson Prentice Hall
Buffers
Animation 26
Discover the chemistry behind buffer action.
Slide
145 of 35
© Copyright Pearson Prentice Hall
19.5
Buffers
Slide
146 of 35
© Copyright Pearson Prentice Hall
Slide
147 of 35
© Copyright Pearson Prentice Hall
Slide
148 of 35
© Copyright Pearson Prentice Hall
Conceptual Problem 19.2
Slide
149 of 35
© Copyright Pearson Prentice Hall
for Conceptual Problem 19.2
Problem Solving 19.39
Solve Problem 39 with the help of
an interactive guided tutorial.
Slide
150 of 35
© Copyright Pearson Prentice Hall
19.5 Section Quiz.
1. Which of the following reactions would most
likely yield a basic salt solution?
a. strong acid + weak base
b. weak acid + weak base
c. strong acid + strong base
d. weak acid + strong base
Slide
151 of 35
© Copyright Pearson Prentice Hall
19.5 Section Quiz.
2. Choose the correct words for the spaces. A
buffer can be a solution of a _________ and
its _________.
a. weak acid, salt
b. strong acid, salt
c. weak acid, conjugate base
d. weak base, conjugate acid
Slide
152 of 35
© Copyright Pearson Prentice Hall






