Blood lecture_7_5_06
advertisement

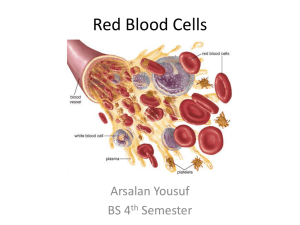
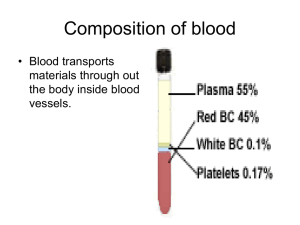
Forensic Biology Screening Workshop Blood What Is Blood? • Slightly alkaline fluid made up of water, cells, enzymes, proteins, glucose, hormones, organic and inorganic substances • Circulates throughout body – Supplies nutrients and oxygen to body – Removes waste Blood Cells • Blood cells are made in the bone marrow – Hemopoiesis – Starts as a “stem cell” (blasts) • Some immature blood cells stay in the bone marrow to mature • Others travel to other parts of the body to mature (lymph nodes, spleen, liver) Blood Cells • Cells mature and differentiate into several classes of cells: – Red blood cells – White blood cells – Platelets Red Blood Cells (Erythrocytes) • Have no nucleus – Not useful for DNA analysis • 6-8 µm in size • ~45% total volume of blood • Most abundant cell in the blood Red Blood Cells – Disk shaped cells which make up 99% of the cells in the blood – Principal carriers of the red colored hemoglobin molecules. • Hemoglobin is an iron containing protein and binds about 97% of all oxygen in the body. Red Blood Cells Hemoglobin • Contains 4 subunits • Globular proteins with heme • Heme groups contain iron which binds O2 • Arterial Blood: bright red when hemoglobin carries O2 • Venous Blood: dark red (veins appears blue in color due to optical effects of light when hemoglobin lacks O2) Hemoglobin Heme With Iron White Blood Cells (WBC) (Leukocytes) • Produced in bone marrow • WBCs have a nucleus – Useful for DNA analysis • Vital source of defense against external organisms • White blood cells also clean up dead cells and tissue debris that would otherwise accumulate and lead to problems. White Blood Cells • Five classes of leukocytes: – Neutrophil – Eosinophil – Basophil – Monocyte – Lymphocyte Red and White Blood Cells image courtesy of the U.S. National Institutes of Health Platelets • Irregularly-shaped, colorless bodies produced in the bone marrow • Their sticky surface lets them, along with other substances, form clots to stop bleeding • Only active when damage occurs to the circulatory system walls Hemostasis Plasma • Liquid portion of blood – Composed of water, proteins, electrolytes – blood cells and platelets are suspended in plasma • Regulates osmotic pressure • The transport medium for: • • • • • • Glucose Lipids Hormones Oxygen (not as much as heme) Clotting factors Waste Serum • Clear liquid that is left after blood coagulates • Plasma without the clotting factors Forensic Significance Of Blood • Hemoglobin (RBC) • Peroxidase-like activity can cleave H2O2 • Blood Group Antigen (RBC) • Bound to RBC membrane (ABO groups) • DNA (WBC) • Found in cells with nucleus • Proteins (PLASMA) • Serum used in species testing Forensic Testing • Presumptive tests – Indicates a substance is present – Not specific • Confirmatory tests – Confirm a substance is present – Specific Presumptive Tests • • • • • Kastle-Meyer (Phenolphthalein) Leucomalachite Green (LMG) Hemastix Luminol Other tests: • Tetramethylbenzidine (TMB) • Benzidine • Ortho-tolidine • Ortho-toluidine Presumptive Tests CATALYTIC TESTS – Based on the fact that hemoglobin (and some of its derivatives) exhibit a peroxidase activity • Tests based on this property are generally named after the compound undergoing oxidation (benzidine, TMB, etc.) or the discoverer (KastleMeyer, etc.) – Oxidant (hydrogen peroxide) oxidizes a colorless reagent to a colored reagent – Heme catalyzes this oxidation by cleaving an oxygen from hydrogen peroxide (H2O2) Kastle-Meyer (KM) Reaction Kastle-Meyer Test HOW TO MAKE: • Phenolphthalein + Potassium Hydroxide + Zinc • Reflux – boil / condense • Reduces phenolphthalein to phenolphthalin –Oxygen removed and combined with OH from potassium hydroxide – boils off as water –Becomes a colorless solution • Working solution • Phenolphthalin + ethanol + zinc pellets Kastle-Meyer Test HOW TO STORE: – Amber bottle • Light affects stability – Zinc pellets • Binds free oxygen, prevents oxidation – Room temp (working solution) – Refrigerate (stock solution) Kastle-Meyer Test HOW TO PERFORM: • Place a small cutting, swabbing, or extract of the suspected bloodstain on filter paper - OR - • Swab the stain using a slightly moistened swab Kastle-Meyer Test 3 STEP TEST STEP 1: Add 2-3 drops of ethanol to the stain or swabbing Note: This will increase sensitivity by cleaning the area around the hemoglobin, better exposing the heme Kastle-Meyer Test STEP 2: Add 2 drops of reagent •Wait for ~5 seconds Note: This step aids in ruling out false positives due to the presence of chemical oxidants such as rust. Kastle-Meyer Test STEP 3: Add 2-3 drops of 3% H2O2 • If immediate color change to PINK – the test is POSITIVE for the possible presence of blood • If no color change – blood is not present or is in too limited quantity for the test to detect. Note: swab will eventually turn pink (even if negative) over time due to nature of oxidation reactions. Kastle-Meyer Test LIMITATIONS • Sensitivity – 1 in 105 to 1 in 106 on dried stains • Specificity – Can weed out false positives between steps 2 and 3 – Chemical oxidants, vegetable peroxidases – Will not detect differences in animal or human blood • Stability – Relatively stable if the reagents are stored separately and refrigerated. Kastle-Meyer Video Leucomalachite Green (LMG) Reaction Leucomalachite Green HOW TO MAKE: • Malachite green + acetic acid + water + Zinc • Reflux – boil / condense –Becomes a colorless solution • Working solution • LMG reagent + zinc pellets Leucomalachite Green • HOW TO STORE: – Amber bottle – Zinc pellets – Relatively stable at room temp Leucomalachite Green HOW TO PERFORM: • Place a small cutting, swabbing, or extract of the suspected bloodstain on filter paper - OR - • Swab the stain using a slightly moistened swab Leucomalachite Green HOW TO PERFORM: STEP 1: Add 1-2 drops of LMG reagent Note color change, if there is a color change, the test is considered inconclusive Leucomalachite Green HOW TO PERFORM: STEP 2: Add 1-2 drops of H2O2 Note results – If color change to deep green-blue, the test is positive for the possible presence of blood – If no color change the test is negative Leucomalachite Green LIMITATIONS: • Sensitivity – ~1:1000 • Specificity – Chemical oxidants, vegetable peroxidases – Will not detect differences in animal or human blood • Stability – Similar to KM Leucomalachite Green Video Hemastix • Reagent strips • Bottle of 50 reagent strips – Store at room temp • Test is based on the peroxidase activity of hemoglobin Hemastix • Reagent on hemastix is diisopropylbenzene dihydroperoxide and 3,3’,5,5’-tetramethylbenzidine (TMB) • Color change ranges from orange to green – Possibly blue with higher concentrations of blood Hemastix HOW TO PERFORM: STEP 1: Slightly moisten the pad on the tip of the strip with water STEP 2: Rub the damp pad on the stain in question Note color change within 60 seconds and compare to the chart on the bottle • more green indicates more hemoglobin Hemastix LIMITATIONS • Sensitivity = 0.015-0.062 mg/dL free hemoglobin • Specificity – Chemical oxidants, vegetable peroxidases – Will not detect differences in animal or human blood Stability – Stable for ~ 1 year – date stamped expiration date on bottle Hemastix Video Luminol Luminol • HOW IT WORKS: – The iron in hemoglobin acts as a catalyst to cause a reaction between the luminol and H2O2. – Luminol loses nitrogen and hydrogen and gains oxygen. – This results in 3-aminopthalate which is energized and emits light Luminol • HOW TO MAKE: – Reagents needed: • Luminol (3-aminophthalhydrazide) • Sodium Perborate • Distilled water • Sodium Carbonate Luminol • HOW TO STORE: – Spray bottle works best for testing – Make each time you use it (daily) Luminol HOW TO PERFORM: STEP 1: Spray the luminol directly onto the stain in question Note: This test needs to be done in the dark to see the luminescence reaction which can last for approximately 15 seconds –If the stain emits a light then the test result is POSITIVE for the possible presence of blood –If there is no reaction the result is NEGATIVE Luminol LIMITATIONS: • Sensitivity – 10-6 to 10-8 – most sensitive presumptive test • Specificity – Many false positives – bleach, metals, chemical oxidants, vegetable peroxidases – Will not detect differences in animal or human blood • Stability – Very unstable ~8 hour limit • Mostly used at crime scene – Can dilute out stain (possibly too much for DNA analysis) – Used more for blood spatter, crime scene reconstruction Tetramethylbenzidine (TMB) Tetramethylbenzidine (TMB) HOW TO MAKE: • 3,3’, 5,5 ‘-tetramethylbenzidine (TMB) + glacial acetic acid • Easy to make compared to KM and LMG reagents Tetramethylbenzidine (TMB) HOW TO STORE: – Amber bottle • Light affects stability – Refrigerate between uses – only good for ~ 1 week. Tetramethylbenzidine (TMB) HOW TO PERFORM: • Place a small cutting, swabbing, or extract of the suspected bloodstain on filter paper - OR - • Swab the stain using a slightly moistened swab Tetramethylbenzidine (TMB) HOW TO PERFORM: STEP 1: Add one drop of TMB solution STEP 2: Add one drop of 3% H2O2 Detect color change: • If the stain turns blueish green, the test result is POSITIVE for the possible presence of blood • NEGATIVE if no color change Tetramethylbenzidine (TMB) LIMITATIONS • Sensitivity – 1:10,000 on dried stains • Specificity – Not as specific as KM test – False positives to vegetable peroxidases, bleach, potassium permanganate – Will not detect differences in animal or human blood Tetramethylbenzidine (TMB) LIMITATIONS • Stability – Very unstable – 1 week maximum – Loses sensitivity by a factor of 10 after 1 day • Safety – Mutagen Other Tests • Benzidine • Ortho tolidine (o-tolidine) - 3,3'Dimethylbenzidine – Increased sensitivity, decreased specificity, and same stability when compared to KastleMeyer – Rarely used due to safety concernscarcinogenic Species Testing Definitions: • Antigen (Ag) – a soluble protein that causes the production of an antibody. Usually foreign to the body. • Antibody (Ab) – A “Y” shaped immunoglobulin produced by B-lymphocytes in the bone marrow. – Produced in response to an antigen. Species Testing • Ag and Ab bind together to create a lattice network of molecules • This becomes insoluble and precipitates out of solution Species Testing To make the antibodies: –Host animal – Goat, rabbit –Inject with human serum –Makes antibodies in response –Collect heart blood and test for Ab Sample extracts are made from stained areas of interest, and from nearby unstained areas (substrate controls). Note that the use of unstained controls is a fundamental principle in forensic immunologic testing. Tube Method • The original precipitin reaction was carried out by layering a solution of antibody on top of a solution of stain extract in a tube • This mixture was left for a period of time to allow the development of a precipitin band at the interface. • This is referred to as the tube method, and is still used in a few laboratories today Ouchterlony Method published in 1948 and uses radial diffusion of the antigen and antibody through agar gel. How to perform: •Extract the stain in a microcentrifuge tube with saline. Extract should be straw colored. •Stain and controls samples are loaded in the outer wells and a drop of anti-human antiserum is loaded into the center well. The process is repeated for antisera to other species, such as dog, cat, and cow; this may include the species from which the antiserum was obtained (e.g., rabbit). Ouchterlony • The plates are left at room temperature or 37°C for a suitable period (which can range from a few hours to overnight) and the serum proteins and antibody molecules diffuse outward from the wells. • A precipitin band is formed when the diffusing stain contains proteins that are recognized by IgG molecules in the diffusing antiserum. • The precipitin band is sometimes clearly visible to the naked eye, but it is normal to stain the plates with amido black, Coomassie Blue, or other general protein stain, to enhance sensitivity and clarity. Ouchterlony – If a white line occurs between the samples, then the test sample is positive for human antigen activity (or whatever species is being tested) – If no white line occurs then the test sample is negative for human antigen activity (or whatever species is being tested) Ouchterlony • Sensitivity – 10-1 • Specificity – False positives: »Detergents »Tannic Acid – False negatives: »Excess Ab or Ag – the lattice will not form, no precipitate Ouchterlony Ouchterlony Video Cross-over Electrophoresis • A variant of the Ouchterlony test, which uses an electric field rather than diffusion to move the extract and antibody through the gel. • Cross-over electrophoresis for species identification is conducted using agar at a pH of 8.6. • Stain extracts are loaded into wells arranged in a line at the cathode end of the plate and the antiserum is loaded into wells at the anode end. Cross-over Electrophoresis • During electrophoresis, the electric field drives the serum proteins towards the anode, but the IgG molecules, which are essentially neutral at this pH, are driven to the cathode by the process of electroendosmosis. • The antigen-antibody precipitation occurs at the interface between the two rows of wells. Cross-over Electrophoresis • Electroendosmosis occurs because the supporting medium acquires a net negative charge. – If free, the negatively charged molecules would migrate to the anode, but this is not possible because the agar is immobilized on the plate. – Instead, the effect is countered by positively charged water molecules migrating to the cathode. The migrating water molecules carry any dissolved neutral molecules (such as IgG) with them. ABAcard® Hematrace® • Tests for human hemoglobin (Hb) • If human Hb is present – reacts with a mobile monoclonal anti-human HB antibody • Forms a mobile Ag-Ab complex • This migrates to the “T” zone ABAcard® Hematrace® • In the “T” zone – polyclonal antihuman HB antibodies. • Forms Ab-Ag-Ab complex • Antibodies tagged with pink dye – upon aggregation at “T” zone – pink line • Control zone – has immobile antiimmunoglobulin which binds excess antihuman HB antibodies – form a pink line ABAcard® Hematrace® To perform: • Extract cutting from stain in ~300 µl buffer • Leave 1-5 minutes • Add 150 µl of sample to the “S” well of the test card • Wait 10 minutes – read results • Pink line in “T” and “C” zones = POSITIVE • Pink line in only “C” zone = NEGATIVE ABAcard® Hematrace® Limitations: • High Dose Hook Effect – can give a false negative – Occurs with excess hemoglobin which binds to the stationary antihuman HB Antibody in the “T” area – This prevents the mobile Ab-Ag complex from binding – Results in no pink line = NEGATIVE – FALSE NEGATIVE • Not a confirmatory test – gives positive result with ferret blood ABAcard® Hematrace® Hematrace Video Seratec HemDirect Hemoglobin Assay Tests for human hemoglobin (Hb) • Similar to the ABAcard® Hematrace® test Hematin Crystals • Teichmann published method in 1853 • Blood is treated with a solution of potassium bromide, potassium chloride and potassium iodide in glacial acetic acid, and is heated to react with hemoglobin. The reaction first converts the hemoglobin to hemin, and then the halides react with the hemin to form characteristic brownish-yellow rhomboid crystals. • The test went through many modifications • Test did not always work even when the presence of blood was known Hemochromagen Crystals • Many variations were used • Takayama described his method in 1912, and it became the preferred method. His name is commonly used to describe the reagents and procedure he devised and also the hemochromogen crystals obtained • Blood is treated with a saturated dextrose solution, sodium hydroxide, pyridine and water. Ferrous iron from hemoglobin reacts with pyridine to produce red feathery crystals of pyridine ferroprotoporphyrin Takayama Crystals image courtesy Steve Gilbert, M.F.S., PhD -- SUNY at Canton Crystal Tests – Crystals are unique to blood – Not human specific – Not widely used anymore –Combo KM and ABA card –Time/money constraints –DNA testing is higher primate specific –Use of qPCR Controls • Positive • Negative • Substrate Positive Controls • Used to determine if the tests are working properly – a quality control check • This is documented in the case file Example: Known blood on cloth or a swab Negative Controls • Used to determine if the reagents are contaminant free and working properly – a quality control check • This is documented in the case file Example: unstained cloth or a swab Substrate Controls • Used to determine if the substrate is interfering with the test – troubleshooting • This is documented in the case file Example: unstained area adjacent to the questioned stain being tested