NLO - School of Chemistry
advertisement
Introduction to Nonlinear
Optical Effects and Materials
T.P.Radhakrishnan
School of Chemistry, University of Hyderabad
Hyderabad 500 046, India
tprsc@uohyd.ernet.in
http://chemistry.uohyd.ernet.in/~tpr/
This file is available at http://chemistry.uohyd.ernet.in/~ch521/
Nonlinear Optical Processes and Materials
Linear optical processes
Reflection, Refraction, Absorption
No change in properties of medium or light
Nonlinear optical processes
Electro-optic effect (Pockel's effect)
- (2) (-;,0)
Electro-optic modulators
Frequency doubling
- (2) (-2;,)
Harmonic generation
Frequency mixing
- (2) (-0;a,b)
Parametric amplifiers
Frequency tripling
Deep UV conversion
- (3) (-3;,,)
Nonlinear Optical Materials
Inorganic crystals (KDP, LiNbO3)
Molecular Solids
Conjugated Polymers
P
Nonlinear polarisation
Linear polarisation
E
P
P
E
E
time
time
Bulk Polarisation
Pi = ij(1) Ej + ijk(2) Ej Ek + ijkl(3) Ej Ek El + ….
Molecular Polarisation
i = ij Ej + ijk Ej Ek + ijkl Ej Ek El + ….
First hyperpolarisability
Centrosymmetric systems
E -E,
P -P
(2) , = 0
Noncentrosymmetric systems
E -E,
P P'
(2) , 0
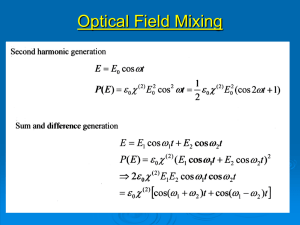
Second Harmonic Generation (SHG)
E sin (t)
P(2)
sin2(t)
{ 1 - cos(2t)}
Quadratic Nonlinear Optical (NLO) Effect :
Second harmonic generation (Frequency doubling)
1064 nm (infra red laser)
532 nm (green laser)
Symmetry condition :
No centre of inversion in the material
Resonance enhancement of SHG :
DE ~ hν or 2hν
Unsymmetric polarisation of a
donor-acceptor substituted benzene
D-
A+
1
D
A
2
A-
D+
3
2-level
3e 2 2
=
2m
f. D. DE
2
2
2
[( DE - 2h ) ( DE - (h ) )]
3e2h2 f. D
0 =
3
2m
DE
Oriented Gas Model
(2)IJK = NfI(2) fJ() fK() bIJK
Ng
bIJK = {cos Ii(s) cos Ii(s) cos Ii(s)} ijk
ijk s=1
Design of Noncentrosymmetric Molecular Materials
Molecular Crystals
H-bonding
Alkyl Chain Effect
Cancelling µ
Steric Effect
Salt Formation
Chirality
Langmuir-Blodgett Films
Poled Thin Films, Polymers
Host-Guest Systems and Intercalation
H-bonding
Urea
-
8 atom molecule
8 H-bonds per molecule !
Melting point = 136oC
Hygroscopic
H-bond
O
O-
Vanishing
Ground
Vanishing Ground
State Dipole
Moment
(and Steric Factor)D donor group
.01 D
O-
State Dipole Moment
4.01 D
D+
NO 2
O-
NO 2
CH 3
A-
A acceptor group
D > 0
Centrosymmetric
+
+
+
N
N
O-
O
N
N
O-
O-
O-
O-
4.24 D
4.01 D
NO 2
D+
D donor group
+
+
N
D < 0
N
NO 2
P212121
Noncentrosymme
Pmna
Centrosymmetric
CH 3
D > 0
+
N
N
O-
O-
+
+
N
N
SHG = 13 U
O-
4.24 D
NO 2
3-methyl-4-nitropyridine
-1-oxide (POM)
O-
Nonentrosymmetric
NO 2
Pmna
CH 3
P212121
Noncentrosymmetric
Organic Salts
Molecular dipoles of a typical
covalent compound
+
+
+
+
+
+
+
+
Molecular dipoles and counterions
in an organic salt
(CH3)2N
+
N
H3C
-
SO3
CH3
Cc (noncentrosymmetric)
SHG = 1000 U
Chirality
Chiral object lacks Sn symmetry
Eg.
S1 ,
S2 i
Crystal of pure enantiomer has to be
noncentrosymmetric
Pure Enantiomer Crystal with no inversion symmetry
Chirality and
H-bonding
H3C
H
COOCH3
NH
NO2
(2,4-dinitrophenyl-Lalanine methyl ester (MAP)
P21 space group
SHG = 10 U
NO2
Chirality and extended H-bonding
H
N
NO2
CH2OH
N-(4-nitrophenyl)-Lprolinol (NPP)
P21 space group
SHG = 150 U
Organic Molecules with Large Second
Order Nonlinearities
Molecule
Powder SHG / U Melting Point /
o
(1 U ~ 3 KDP)
C
O
OMe
H
N
O2N
*
10
81
40
114
80
131
115
166
150
116
NO2
MAP
NH2
NO2
mNA
NH2
CH3
NO2
MNA
N
NH-CO-CH 3
NO 2
DAN
CH 2OH
*
N
NO 2
NPP
Kurtz-Perry Powder SHG Measurement
1064 nm
M
F532
532 nm
Mono
F1064
S
PC
Osc
1064 nm : Nd:YAG laser
F532 : green filter
F1064 : interference (ir) filter
M : concave mirror
S : microcrystalline sample
Mono : monochromator
Osc : oscilloscope
Electric Field Induced Second Harmonic
Generation (EFISHG)
Cell Design
Electric Field Induced
Second Harmonic Generation
(EFISHG)
Refractive Index variation
n = n0 + n2I
Pockel’s effect (2nd order effect)
Refractive index varies with applied dc field
Kerr effect (3rd order effect)
Refractive index varies with incident laser intensity
Kerr gate
Cross
Polarizer
Polarizer
Laser
No light
(3) crystal
Laser 2
Cross
Polarizer
Polarizer
Laser 1
Light
(3) crystal