Ch#14 Acids and Bases
advertisement

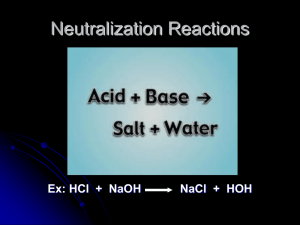
Chapter #14 Acids, Bases, and Salts Acids, Bases and Salts Topics The Arrhenius Theory The Brønsted Theory Naming Acids (See Nomenclature Notes) The Self-Ionization of Water The pH Concept Properties of Acids Properties of Bases Salts The Strengths of Acids and Bases Analyzing Acids and Bases Titration Calculations Hydrolysis Reactions of Salts Buffers History of Acids and Bases In the early days of chemistry chemists were organizing physical and chemical properties of substances. They discovered that many substances could be placed in two different property categories: Substance B Substance A 1. Bitter taste 1. Sour taste 2. Reacts with carbonates to make CO2 2. 3. Reacts with metals to produce H2 3. Do not react with metals 4. Turns blue litmus pink 4. Turns red litmus blue 5. Reacts with B substances to make salt water 5. Reacts with A substances make salt and water Reacts with fats to make soaps Arrhenius was the first person to suggest a reason why substances are in A or B due to their ionization in water. Arrhenius Theory The Swedish chemist Svante Arrhenius proposed the first definition of acids and bases. (Substances A and B became known as acids and bases) According to the Arrhenius model: “acids are substances that dissociate in water to produce H+ ions and bases are substances that dissociate in water to produce OH- ions” NaOH (aq) Na+ (aq) + OH- (aq) Base HCl (aq) H+ (aq) + Cl- (aq) Acid What is + H? e- + Hydrogen (H) + Proton (H+) Hydronium Ion Unknown to Arrhenius free H+ ions do not exist in water. They covalently react with water to produce hydronium ions, H3O+. or: H+ (aq) + H2O (l) H3O+ (aq) This new bond is called a coordinate covalent bond since both new bonding electrons come from the same atom Hydronium Ion Hydronium ion is the name for H3O+ and is often times abbreviated as H+ (aq) they both mean the same thing. What is the difference between a strong acid and a weak acid? Hydronium Ion Hydronium ion is the name for H3O+ and is often times abbreviated as H+ (aq) they both mean the same thing. What is the difference between a strong acid and a weak acid? Strong acids ionize 100% and weak ones do not! Hydronium Ion Hydronium ion is the name for H3O+ and is often times abbreviated as H+ (aq) they both mean the same thing. What is the difference between a strong acid and a weak acid? Strong acids ionize 100% and weak ones do not! A single arrow is used to represent the ionization of a strong acid. Double arrows (Equilibrium) are used to represent weak acids. For example: HCl (g) H+ (aq) + Cl - (aq) HF (g) H+ (aq) + F - Hydronium Ion Hydronium ion is the name for H3O+ and is often times abbreviated as H+ (aq) they both mean the same thing. What is the difference between a strong acid and a weak acid? Strong acids ionize 100% and weak ones do not! A single arrow is used to represent the ionization of a strong acid. Double arrows (Equilibrium) are used to represent weak acids. For example: HCl (g) H+ (aq) + Cl - (aq) HF (g) H+ (aq) + F - According to Arrhenius, is water an acid or base? HOH (l) H+ (aq) + OH – (aq) Hydronium Ion Hydronium ion is the name for H3O+ and is often times abbreviated as H+ (aq) they both mean the same thing. What is the difference between a strong acid and a weak acid? Strong acids ionize 100% and weak ones do not! A single arrow is used to represent the ionization of a strong acid. Double arrows (Equilibrium) are used to represent weak acids. For example: HCl (g) H+ (aq) + Cl - (aq) HF (g) H+ (aq) + F - According to Arrhenius, is water an acid or base? HOH (l) H+ (aq) + OH – (aq) Neither, he called it Neutral (same amount of OH- and H+ Strong Acids and Bases How can we identify strong acids or bases? Strong Acids and Bases How can we identify strong acids or bases? Easy memorize them! Strong Acids and Bases How can we identify strong acids or bases? Easy, memorize them! Memorized Strong Acids 1. HClO4 2. H2SO4 3. HI 4. HBr 5. HCl 6. HNO3 Memorized Strong Bases Hydroxides of group 1 and 2 metals, excluding Be and Mg Bronsted Lowry Theory Johannes Brønsted and Thomas Lowry revised Arrhenius’s acid-base theory to include this behavior. They defined acids and bases as follows: Bronsted Lowry “An acid is a hydrogen containing species that donates a proton. A base is any substance that accepts a proton” HCl (aq) + H2O (l) Cl- (aq) + H3O+ (aq) In the above example what is the Brønsted acid? What is the Brønsted base? Bronsted Lowry Theory In reality, the reaction of HCl with H2O is an equilibrium and occurs in both directions, although in this case the equilibrium lies far to the right. HCl (aq) + H2O (l) Cl - ( aq) + H3O+ (aq) For the reverse reaction Cl - behaves as a Brønsted base and H3O+ behaves as a Brønsted acid. The Cl- is called the conjugate base of HCl. Brønsted acids and bases always exist as conjugate acid-base pairs. Autoionization of Water In pure water (no solute) water molecules behave as both an acid and base!! e.g. H2O (l) + H2O (l) H3O+ (aq) + OH- (aq) This is called the self-ionization (autoionizaion) of water. Although the equilibrium lies far to the left it is very important to take into consideration, especially for living systems. Does anyone know how we write the equilibrium constant for this reaction? Autoionization of Water The auto-ionization of water is described by the equation: H2O (l) + H2O (l) H3O+ (aq) + OH- (aq) The equilibrium constant for this reaction is given by: [H3O ][ OH ] [H3O ][ OH ] K [H2O][H2O ] [H2O ]2 K[H2O ]2 [H3O ][ OH ] Kw = K[H2O]2 = 10-14 This equilibrium lies very much to the left i.e. mostly water. For pure water [OH-] = [H+] = 1 x 10-7 M Autoionization of Water As [OH-] and [H+] are so small the [H2O] is not affected by their formation. It is useful to define a new constant Kw such that: 1.00 g ml mole 18.0 g ml 10-3 L = 55.5 M [H3O ][ OH ] [H3O ][ OH ] K [H2O ][H2O ] [H2O ]2 K[H2O ]2 K w [H3O ][ OH ] Kw is called the ion product of water. What is the value for the ion product of water? Autoionization of Water As [OH-] and [H+] are so small the [H2O] is not affected by their formation. It is useful to define a new constant Kw such that: 1.00 g ml mole 18.0 g ml 10-3 L = 55.5 M [H3O ][ OH ] [H3O ][ OH ] K [H2O ][H2O ] [H2O ]2 K[H2O ]2 K w [H3O ][ OH ] Kw is called the ion product of water. What is the value for the ion product of water? [H+][OH-] = 10-14 Autoionization of Water We define an aqueous solution as being neutral when the [H+] = [OH-]. We define an aqueous solution as being acidic when [H+] > [OH-]. We define an aqueous solution as being basic when [H+] < [OH-]. However, in each case Kw = 1 x 10-14 M2 [H+] = 0.0000001 = 10-7 (how can this be abbreviated further?) Autoionization of Water We define an aqueous solution as being neutral when the [H+] = [OH-]. We define an aqueous solution as being acidic when [H+] > [OH-]. We define an aqueous solution as being basic when [H+] < [OH-]. However, in each case Kw = 1 x 10-14 M2 [H+] = 0.0000001 = 10-7 (how can this be abbreviated further?) By just describing the power Autoionization of Water We define an aqueous solution as being neutral when the [H+] = [OH-]. We define an aqueous solution as being acidic when [H+] > [OH-]. We define an aqueous solution as being basic when [H+] < [OH-]. However, in each case Kw = 1 x 10-14 M2 [H+] = 0.0000001 = 10-7 (how can this be abbreviated further?) By just describing the power Called the power of H, or pH. Autoionization of Water We define an aqueous solution as being neutral when the [H+] = [OH-]. We define an aqueous solution as being acidic when [H+] > [OH-]. We define an aqueous solution as being basic when [H+] < [OH-]. However, in each case Kw = 1 x 10-14 M2 [H+] = 0.0000001 = 10-7 (how can this be abbreviated further?) By just describing the power Called the power of H, or pH. Our math departments tells us that log means pH = 7 power too. The mathematical definition of pH using [H+] for [H3O+] is listed below: pH = -log [H+], or [H+]= 1x10-pH (both are mathematically equivalent) How about the power for the OH -, what should this be called? Autoionization of Water The mathematical definition of pH using [H+] for [H3O+] is listed below: pH = -log [H+], or [H+] = 1x10-pH (both are mathematically equivalent) How about the power for the OH -, what should this be called? Would you believe pOH? Autoionization of Water The mathematical definition of pH using [H+] for [H3O+] is listed below: pH = -log [H+], or [H+]= 1x10-pH (both are mathematically equivalent) How about the power for the OH -, what should this be called? Would you believe pOH? Have you heard of pOH before? Autoionization of Water The mathematical definition of pH using [H+] for [H3O+] is listed below: pH = -log [H+], or [H+]= 1x10-pH (both are mathematically equivalent) How about the power for the OH -, what should this be called? Would you believe pOH? Have you heard of pOH before? pH + pOH = 14 for water solutions. Now for some examples 1. Find the pH and pOH, when [H+] = 10-4 Now for some examples 1. Find the pH and pOH, when [H+] = 10-4 pH = 4 and pOH = 10, since they must add to 14 using the calculator pH = -log [H+], type in 10-4, push the log button and pH = -(-4) = 4. Same for pOH A pH Number line Number lines have been used in history and math classes, so to keep up we use them in chemistry classes. pH = 16 pH = 12 pH = 7 [H+] = 10-2 pH = 2 A pH Number line Number lines have been used in history and math classes, so to keep up we use them in chemistry classes. pH = 16 pH = 12 pH = 7 pH = 2 [H+] = 10-2 [OH -] = 10-12 A pH Number line Number lines have been used in history and math classes, so to keep up we use them in chemistry classes. pH = 16 pH = 12 pH = 7 pH = 2 [H+] = 10-2 [OH -] = 10-12 [H+] > [OH -] acidic A pH Number line Number lines have been used in history and math classes, so to keep up we use them in chemistry classes. pH = 16 pH = 12 pH = 7 [H+] = 10-7 [OH -] = 10-7 pH = 2 [H+] = 10-2 [OH -] = 10-12 [H+] > [OH -] acidic A pH Number line Number lines have been used in history and math classes, so to keep up we use them in chemistry classes. pH = 16 pH = 12 pH = 7 [H+] = 10-7 [OH -] = 10-7 [H+] = [OH -] neutral pH = 2 [H+] = 10-2 [OH -] = 10-12 [H+] > [OH -] acidic A pH Number line Number lines have been used in history and math classes, so to keep up we use them in chemistry classes. pH = 16 pH = 12 [H+] =10-12 [OH -] = 10-2 pH = 7 [H+] = 10-7 [OH -] = 10-7 [H+] = [OH -] neutral pH = 2 [H+] = 10-2 [OH -] = 10-12 [H+] > [OH -] acidic A pH Number line Number lines have been used in history and math classes, so to keep up we use them in chemistry classes. pH = 16 pH = 12 [H+] =10-12 [OH -] = 10-2 pH = 7 [H+] = 10-7 [OH -] = 10-7 [H+] = [OH -] neutral pH = 2 [H+] = 10-2 [OH -] = 10-12 [H+] > [OH -] acidic [H+] < [OH -] basic A pH Number line Number lines have been used in history and math classes, so to keep up we use them in chemistry classes. [H+] =10-16 pH = 16 [OH -] = pH = 12 [H+] =10-12 [OH -] = 10-2 pH = 7 [H+] = 10-7 [OH -] = 10-7 [H+] = [OH -] neutral pH = 2 [H+] = 10-2 [OH -] = 10-12 [H+] > [OH -] acidic [H+] < [OH -] basic A pH Number line Number lines have been used in history and math classes, so to keep up we use them in chemistry classes. [H+] =10-16 pH = 16 [OH -] = 102 pH = 12 [H+] =10-12 [OH -] = 10-2 pH = 7 [H+] = 10-7 [OH -] = 10-7 [H+] = [OH -] neutral pH = 2 [H+] = 10-2 [OH -] = 10-12 [H+] > [OH -] acidic [H+] < [OH -] basic A pH Number line Number lines have been used in history and math classes, so to keep up we use them in chemistry classes. [H+] =10-1 pH = 16 [OH -] = 102 [H+] < [OH -] basic pH = 12 [H+] =10-12 [OH -] = 10-2 pH = 7 [H+] = 10-7 [OH -] = 10-7 [H+] = [OH -] neutral pH = 2 [H+] = 10-2 [OH -] = 10-12 [H+] > [OH -] acidic [H+] < [OH -] basic A pH Number line Number lines have been used in history and math classes, so to keep up we use them in chemistry classes. [H+] =10-16 pH = 16 [OH -] = 102 [H+] < [OH -] basic pH = 12 [H+] =10-12 [OH -] = 10-2 pH = 7 [H+] = 10-7 [OH -] = 10-7 [H+] = [OH -] neutral pH = 2 [H+] = 10-2 [OH -] = 10-12 [H+] > [OH -] acidic acidic [H+] < [OH -] basic A pH Number line Number lines have been used in history and math classes, so to keep up we use them in chemistry classes. [H+] =10-16 pH = 16 [OH -] = 102 basic [H+] < [OH -] basic pH = 12 [H+] =10-12 [OH -] = 10-2 pH = 7 [H+] = 10-7 [OH -] = 10-7 [H+] = [OH -] neutral pH = 2 [H+] = 10-2 [OH -] = 10-12 [H+] > [OH -] acidic acidic [H+] < [OH -] basic Equations With Acuids Acids undergo characteristic double replacement reactions with oxides, hydroxides, carbonates and bicarbonates. e.g. 2HCl (aq) + CuO (s) CuCl2 (aq) + H2O (l) 2HCl (aq) + Ca(OH)2 (aq) CaCl2 (aq) + 2H2O (l) 2HCl (aq) + CaCO3 (aq) CaCl2 (aq) + H2O (l) + CO2 (g) 2HC l (aq) + Sr(HCO3)2 (aq) SrCl2 (aq) + 2H2O (l) + 2CO2 (g) Equations With Acuids Bases undergo a double replacement reaction with acids called neutralization: NaOH (aq) + HCl (aq) H2O (l) + NaC l (aq) In words this well known reaction is often described as: “acid plus base = salt plus water” We previously discussed this reaction when describing types of reactions. Ionic Equations (a review) We have discussed the double replacement reactions and ionic equations before. Since the acids and bases undergo double replacement reactions called neutralization reactions, then they can have ionic equations too. e.g. Formula equation: HCl (aq) + NaOH (aq) NaCl (aq) + H2O (l) Ionic equation: H+ (aq) + Cl- (aq) + Na+ (aq) + OH- (aq) Na+ (aq) + Cl- (aq) + H2O (l) Net ionic equation: H+ (aq) + OH- (aq) H2O (l) Acidic Single Replacement Reactions Another property of acids is their reaction with certain metals to produce hydrogen gas, H2 (g). Zn (s) + 2HC l (aq) H2 (g) + ZnCl2 (aq) This is an example of a single replacement reaction and is a redox reaction. Total ionic equation: Zn (s) + 2H+ (aq) + 2Cl- (aq) H2 (g) + Zn2+ (aq) + 2Cl- (aq) Net ionic equation: Zn (s) + 2H+ (aq) H2 (g) + Zn2+ (aq) Salts Salts are the ionic product of an acid base neutralization reaction. Acidic Salts are formed from a strong acid and a weak base. Neutral salts are formed from a strong acid and strong base. Basic salts are formed from a strong base and a weak acid. Give the acid and base the following salts were formed from and label the salts as acidic, basic, or neutral. 1. NaCl 2. NaC2H3O2 3. NH4Cl Salts Salts are the ionic product of an acid base neutralization reaction. Acidic Salts are formed from a strong acid and a weak base. Neutral salts are formed from a strong acid and strong base. Basic salts are formed from a strong base and a weak acid. Give the acid and base the following salts were formed from and label the salts as acidic, basic, or neutral. 1. NaCl 1. NaC2H3O2 1. NH4Cl Reactants are? NaCl + HOH Salts Salts are the ionic product of an acid base neutralization reaction. Acidic Salts are formed from a strong acid and a weak base. Neutral salts are formed from a strong acid and strong base. Basic salts are formed from a strong base and a weak acid. Give the acid and base the following salts were formed from and label the salts as acidic, basic, or neutral. 1. NaCl 2. NaC2H3O2 3. NH4Cl HCl + NaOH s.b. S.A. NaCl + HOH NaC2H3O2 + HOH Salts Salts are the ionic product of an acid base neutralization reaction. Acidic Salts are formed from a strong acid and a weak base. Neutral salts are formed from a strong acid and strong base. Basic salts are formed from a strong base and a weak acid. Give the acid and base the following salts were formed from and label the salts as acidic, basic, or neutral. 1. NaCl Neutral salt 2. NaC2H3O2 3. NH4Cl HCl + NaOH s.b. S.A. NaCl + HOH NaC2H3O2 + HOH Salts Salts are the ionic product of an acid base neutralization reaction. Acidic Salts are formed from a strong acid and a weak base. Neutral salts are formed from a strong acid and strong base. Basic salts are formed from a strong base and a weak acid. Give the acid and base the following salts were formed from and label the salts as acidic, basic, or neutral. 1. NaCl Neutral salt 2. NaC2H3O2 3. NH4Cl HCl + NaOH s.a. s.b. HC2H3O2 + NaOH NaCl + HOH NaC2H3O2 + HOH Salts Salts are the ionic product of an acid base neutralization reaction. Acidic Salts are formed from a strong acid and a weak base. Neutral salts are formed from a strong acid and strong base. Basic salts are formed from a strong base and a weak acid. Give the acid and base the following salts were formed from and label the salts as acidic, basic, or neutral. 1. NaCl HCl + NaOH s.a. Neutral salt 2. NaC2H3O2 HC2H3O2 + NaOH w.a. 3. NH4Cl s.b. s.b. NaCl + HOH NaC2H3O2 + HOH Salts Salts are the ionic product of an acid base neutralization reaction. Acidic Salts are formed from a strong acid and a weak base. Neutral salts are formed from a strong acid and strong base. Basic salts are formed from a strong base and a weak acid. Give the acid and base the following salts were formed from and label the salts as acidic, basic, or neutral. 1. NaCl HCl + NaOH s.a. Neutral salt 2. NaC2H3O2 basic salt 3. NH4Cl s.b. HC2H3O2 + NaOH w.a. s.b. NaCl + HOH NaC2H3O2 + HOH Salts Salts are the ionic product of an acid base neutralization reaction. Acidic Salts are formed from a strong acid and a weak base. Neutral salts are formed from a strong acid and strong base. Basic salts are formed from a strong base and a weak acid. Give the acid and base the following salts were formed from and label the salts as acidic, basic, or neutral. 1. NaCl HCl + NaOH s.a. Neutral salt 2. NaC2H3O2 basic salt 3. NH4Cl s.b. HC2H3O2 + NaOH w.a. NaCl + HOH NaC2H3O2 + HOH s.b. NH4Cl + HOH Salts Salts are the ionic product of an acid base neutralization reaction. Acidic Salts are formed from a strong acid and a weak base. Neutral salts are formed from a strong acid and strong base. Basic salts are formed from a strong base and a weak acid. Give the acid and base the following salts were formed from and label the salts as acidic, basic, or neutral. 1. NaCl HCl + NaOH s.a. Neutral salt 2. NaC2H3O2 basic salt 3. NH4Cl s.b. HC2H3O2 + NaOH w.a. NaCl + HOH NaC2H3O2 + HOH s.b. HCl + NH4OH NH4Cl + HOH Salts Salts are the ionic product of an acid base neutralization reaction. Acidic Salts are formed from a strong acid and a weak base. Neutral salts are formed from a strong acid and strong base. Basic salts are formed from a strong base and a weak acid. Give the acid and base the following salts were formed from and label the salts as acidic, basic, or neutral. 1. NaCl HCl + NaOH s.a. Neutral salt 2. NaC2H3O2 basic salt 3. NH4Cl s.b. HC2H3O2 + NaOH w.a. NaC2H3O2 + HOH s.b. HCl + NH4OH s.a. NaCl + HOH w.b. NH4Cl + HOH Salts Salts are the ionic product of an acid base neutralization reaction. Acidic Salts are formed from a strong acid and a weak base. Neutral salts are formed from a strong acid and strong base. Basic salts are formed from a strong base and a weak acid. Give the acid and base the following salts were formed from and label the salts as acidic, basic, or neutral. 1. NaCl HCl + NaOH s.a. neutral salt 2. NaC2H3O2 basic salt 3. NH4Cl acidic salt s.b. HC2H3O2 + NaOH w.a. NaC2H3O2 + HOH s.b. HCl + NH4OH s.a. NaCl + HOH w.b. NH4Cl + HOH Acid, Base, and Salt Hydrolysis HBr (aq) Acid, Base, and Salt Hydrolysis HBr (aq) H+ (aq) + Br - (aq) Acid, Base, and Salt Hydrolysis HBr (aq) H+ (aq) + Br - (aq) Acidic, because H+ (aq) Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) Initial concentration H+ (aq) + Br - (aq) Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) Initial concentration 0.0 H+ (aq) + Br - (aq) Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) 0.0 H+ (aq) Initial concentration ? + Br - (aq) Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) 0.0 H+ (aq) Initial concentration 0.0 + Br - (aq) Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) ? Initial concentration H+ (aq) + Br - (aq) Final concentration Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) 0.0 Initial concentration H+ (aq) + Br - (aq) Final concentration Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) 0.0 Initial concentration H+ (aq) ? + Br - (aq) Final concentration Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) 0.0 Initial concentration H+ (aq) 0.1 + Br - (aq) Final concentration Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) 0.0 Initial concentration H+ (aq) 0.1 + Br ? - (aq) Final concentration Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) 0.0 Initial concentration H+ (aq) 0.1 + Br 0.1 - (aq) Final concentration Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) 0.0 [H+] = ? Initial concentration H+ (aq) 0.1 + Br 0.1 - (aq) Final concentration Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) 0.0 [H+] = 0.1 Initial concentration H+ (aq) 0.1 + Br 0.1 - (aq) Final concentration Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) 0.0 [H+] = 0.1 pH = ? Initial concentration H+ (aq) 0.1 + Br 0.1 - (aq) Final concentration Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) 0.0 [H+] = 0.1 = 10-1 pH = ? Initial concentration H+ (aq) 0.1 + Br 0.1 - (aq) Final concentration Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) 0.0 [H+] = 0.1 = 10-1 pH = 1 Initial concentration H+ (aq) 0.1 + Br 0.1 - (aq) Final concentration Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) 0.0 Ca(OH)2 (aq) Initial concentration H+ (aq) 0.1 + Br 0.1 - (aq) pH = 1 Final concentration Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) 0.0 Ca(OH)2 (aq) Initial concentration H+ (aq) 0.1 + Br 0.1 - (aq) pH = 1 Final concentration Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) 0.0 Ca(OH)2 (aq) Initial concentration H+ (aq) 0.1 + Br - (aq) 0.1 Ca2+ (aq) + 2 OH- pH = 1 Final concentration (aq) Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) 0.0 Ca(OH)2 (aq) Initial concentration H+ (aq) 0.1 + Br - (aq) 0.1 Ca2+ (aq) + 2 OH- pH = 1 Final concentration (aq) acidic? Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) 0.0 Ca(OH)2 (aq) Initial concentration H+ (aq) 0.1 + Br - (aq) 0.1 Ca2+ (aq) + 2 OH- pH = 1 Final concentration (aq) No, basic OH- Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) 0.0 Initial concentration H+ (aq) 0.1 + Br - (aq) 0.1 0.1 Ca(OH)2 (aq) Ca2+ (aq) + 2 OH- pH = 1 Final concentration Initial concentration (aq) Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) 0.0 0.1 Ca(OH)2 (aq) Initial concentration H+ (aq) 0.1 0.0 + Br - (aq) 0.1 Ca2+ (aq) + 2 OH- pH = 1 Final concentration Initial concentration (aq) Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) 0.0 0.1 Ca(OH)2 (aq) Initial concentration H+ (aq) 0.1 0.0 + Br - (aq) 0.1 pH = 1 Final concentration ? Ca2+ (aq) + 2 OH- Initial concentration (aq) Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) 0.0 0.1 Ca(OH)2 (aq) Initial concentration H+ (aq) 0.1 0.0 + Br - (aq) 0.1 pH = 1 Final concentration 0.0 Ca2+ (aq) + 2 OH- Initial concentration (aq) Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) 0.0 0.1 Ca(OH)2 (aq) ? Initial concentration H+ (aq) 0.1 0.0 + Br - (aq) 0.1 pH = 1 Final concentration 0.0 Ca2+ (aq) + 2 OH- Initial concentration (aq) Final concentration Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) 0.0 0.1 Ca(OH)2 (aq) 0.0 Initial concentration H+ (aq) + Br - (aq) 0.1 0.1 0.0 Final concentration 0.0 Ca2+ (aq) + 2 OH? pH = 1 Initial concentration (aq) Final concentration Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) 0.0 0.1 Ca(OH)2 (aq) 0.0 Initial concentration H+ (aq) + Br - (aq) 0.1 0.1 0.0 Final concentration 0.0 Ca2+ (aq) + 2 OH0.1 pH = 1 Initial concentration (aq) Final concentration Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) 0.0 0.1 Ca(OH)2 (aq) 0.0 Initial concentration H+ (aq) + Br - (aq) 0.1 0.1 0.0 Final concentration 0.0 Ca2+ (aq) + 2 OH0.1 pH = 1 ? Initial concentration (aq) Final concentration Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) 0.0 0.1 Ca(OH)2 (aq) 0.0 Initial concentration H+ (aq) + Br - (aq) 0.1 0.1 0.0 Final concentration 0.0 Ca2+ (aq) + 2 OH0.1 pH = 1 0.2 Initial concentration (aq) Final concentration Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) 0.0 0.1 Ca(OH)2 (aq) 0.0 [OH - ] = ? Initial concentration H+ (aq) + Br - (aq) 0.1 0.1 0.0 Final concentration 0.0 Ca2+ (aq) + 2 OH0.1 pH = 1 0.2 Initial concentration (aq) Final concentration Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) 0.0 0.1 Ca(OH)2 (aq) 0.0 [OH - ] = 0.2 Initial concentration H+ (aq) + Br - (aq) 0.1 0.1 0.0 Final concentration 0.0 Ca2+ (aq) + 2 OH0.1 pH = 1 0.2 Initial concentration (aq) Final concentration Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) 0.0 0.1 Ca(OH)2 (aq) 0.0 [OH - ] = 0.2 pOH = ? Initial concentration H+ (aq) + Br - (aq) 0.1 0.1 0.0 Final concentration 0.0 Ca2+ (aq) + 2 OH0.1 pH = 1 0.2 Initial concentration (aq) Final concentration Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) 0.0 Initial concentration H+ (aq) Ca(OH)2 (aq) Br - (aq) 0.1 0.1 0.1 + 0.0 Final concentration 0.0 Ca2+ (aq) + 2 OH- 0.0 [OH - ] = 0.2 pOH = - log[OH-] 0.1 pH = 1 0.2 Initial concentration (aq) Final concentration Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) 0.0 0.1 Ca(OH)2 (aq) 0.0 Initial concentration H+ (aq) + Br - (aq) 0.1 0.1 0.0 Final concentration 0.0 Ca2+ (aq) + 2 OH0.1 [OH - ] = 0.2 pOH = - log[OH-] = - log[0.2] pH = 1 0.2 Initial concentration (aq) Final concentration Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) 0.0 0.1 Ca(OH)2 (aq) 0.0 Initial concentration H+ (aq) + Br - (aq) 0.1 0.1 0.0 Final concentration 0.0 Ca2+ (aq) + 2 OH0.1 pH = 1 0.2 Initial concentration (aq) Final concentration [OH - ] = 0.2 pOH = - log[OH-] = - log[0.2] = -(-0.698970004) pOH = 0.7 Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) 0.0 0.1 Ca(OH)2 (aq) 0.0 Initial concentration H+ (aq) + Br - (aq) 0.1 0.1 0.0 Final concentration 0.0 Ca2+ (aq) + 2 OH0.1 pH = 1 0.2 Initial concentration (aq) Final concentration [OH - ] = 0.2 pOH = - log[OH-] = - log[0.2] = -(-0.698970004) pOH = 0.7 pH = ? Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) 0.0 0.1 Ca(OH)2 (aq) 0.0 Initial concentration H+ (aq) + Br - (aq) 0.1 0.1 0.0 Final concentration 0.0 Ca2+ (aq) + 2 OH0.1 pH = 1 0.2 Initial concentration (aq) Final concentration [OH - ] = 0.2 pOH = - log[OH-] = - log[0.2] = -(-0.698970004) pOH = 0.7 pH = 14.0 - 0.07 = 13.3 Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) 0.0 0.1 Ca(OH)2 (aq) 0.0 Initial concentration H+ (aq) + Br - (aq) 0.1 0.1 0.0 Final concentration 0.0 Ca2+ (aq) + 2 OH0.1 pH = 1 0.2 Initial concentration (aq) pH = 13.3 final concentration Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) 0.0 0.1 Ca(OH)2 (aq) 0.0 NaF (aq) Initial concentration H+ (aq) + Br - (aq) 0.1 0.1 0.0 Final concentration 0.0 Ca2+ (aq) + 2 OH0.1 pH = 1 0.2 Initial concentration (aq) pH = 13.3 final concentration Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) 0.0 0.1 Ca(OH)2 (aq) 0.0 NaF (aq) Initial concentration H+ (aq) + Br - (aq) 0.1 0.1 0.0 Final concentration 0.0 Ca2+ (aq) + 2 OH0.1 pH = 1 0.2 Na+ (aq) + F – (aq) Initial concentration (aq) pH = 13.3 final concentration Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) 0.0 0.1 Ca(OH)2 (aq) 0.0 NaF (aq) Initial concentration H+ (aq) + Br - (aq) pH = 1 0.1 0.1 0.0 Final concentration 0.0 Ca2+ (aq) + 2 OH0.1 0.2 Na+ (aq) + F – (aq) Initial concentration (aq) pH = 13.3 final concentration Acidic, basic, or neutral? Acid, Base, and Salt Hydrolysis 0.1 HBr (aq) 0.0 0.1 Ca(OH)2 (aq) 0.0 NaF (aq) Initial concentration H+ (aq) + Br - (aq) pH = 1 0.1 0.1 0.0 Final concentration 0.0 Ca2+ (aq) + 2 OH0.1 0.2 Na+ (aq) + F – (aq) Initial concentration (aq) pH = 13.3 final concentration Basic, since HF is w.a. and NaOH is s.b. Will sodium and fluorine ions react with water? Acid, Base, and Salt Hydrolysis 0.1 Initial concentration HBr (aq) 0.0 H+ (aq) Ca(OH)2 (aq) 0.0 NaF (aq) Br - (aq) pH = 1 0.1 0.1 0.1 + 0.0 Final concentration 0.0 Ca2+ (aq) + 2 OH0.1 0.2 Na+ (aq) + F – (aq) Initial concentration (aq) pH = 13.3 final concentration Basic, since HF is w.a. and NaOH is s.b. Will sodium and fluorine ions react with water? Na+ + HOH NaOH (sb) + H+ Acid, Base, and Salt Hydrolysis 0.1 Initial concentration HBr (aq) H+ (aq) 0.0 0.0 Ca(OH)2 (aq) 0.0 Br - (aq) Final concentration 0.0 Ca2+ (aq) + 2 OH0.1 0.2 Na+ (aq) + F – (aq) NaF (aq) pH = 1 0.1 0.1 0.1 + Initial concentration (aq) pH = 13.3 final concentration Basic, since HF is w.a. and NaOH is s.b. Will sodium and fluorine ions react with water? Na+ + HOH NaOH (sb) + H+ Na+ + HOH Na+ + OH- + H+ HOH OH- + H+ No Reaction, water cannot make water Acid, Base, and Salt Hydrolysis 0.1 Initial concentration HBr (aq) 0.0 H+ (aq) Ca(OH)2 (aq) 0.0 NaF (aq) Br - (aq) pH = 1 0.1 0.1 0.1 + Final concentration Initial concentration 0.0 0.0 Ca2+ (aq) + 2 OH0.1 (aq) final concentration 0.2 Na+ (aq) + F – (aq) pH = 13.3 Basic, since HF is w.a. and NaOH is s.b. Will sodium and fluorine ions react with water? Na+ + HOH NaOH + H+ s.b. Cannot make strong acids or bases from weak ones Acid, Base, and Salt Hydrolysis 0.1 Initial concentration HBr (aq) 0.0 H+ (aq) Ca(OH)2 (aq) 0.0 NaF (aq) Br - (aq) pH = 1 0.1 0.1 0.1 + Final concentration Initial concentration 0.0 0.0 Ca2+ (aq) + 2 OH0.1 (aq) final concentration 0.2 Na+ (aq) + F – (aq) pH = 13.3 Basic, since HF is w.a. and NaOH is s.b. Will sodium and fluorine ions react with water? Na+ + HOH NaOH + H+ s.b. Cannot make strong acids or bases from weak ones Acid, Base, and Salt Hydrolysis 0.1 Initial concentration HBr (aq) 0.0 H+ (aq) Ca(OH)2 (aq) 0.0 NaF (aq) Br - (aq) pH = 1 0.1 0.1 0.1 + Final concentration Initial concentration 0.0 0.0 Ca2+ (aq) + 2 OH0.1 (aq) final concentration 0.2 Na+ (aq) + F – (aq) pH = 13.3 Basic, since HF is w.a. and NaOH is s.b. Will sodium and fluorine ions react with water? Na+ + HOH NaOH + H+ F - + HOH HF + OHw.a. Cannot make strong acids or bases from weak ones Acid, Base, and Salt Hydrolysis 0.1 Initial concentration HBr (aq) 0.0 H+ (aq) Ca(OH)2 (aq) 0.0 NaF (aq) Br - (aq) pH = 1 0.1 0.1 0.1 + Final concentration Initial concentration 0.0 0.0 Ca2+ (aq) + 2 OH0.1 (aq) final concentration 0.2 Na+ (aq) + F – (aq) pH = 13.3 Basic, since HF is w.a. and NaOH is s.b. Will sodium and fluorine ions react with water? Na+ + HOH NaOH + H+ F - + HOH HF + OHw.a. Cannot make strong acids or bases from weak ones Yes, HF weak acid and OH- is formed, thus basic salt! Acid, Base, and Salt Hydrolysis NH4Cl (aq) NH4+ (aq) + Cl- (aq) Acid, Base, and Salt Hydrolysis NH4Cl (aq) NH4+ (aq) + Cl- (aq) acidic, basic, or neutral? Acid, Base, and Salt Hydrolysis HCl + NH4OH NH4Cl (aq) NH4Cl + HOH NH4+ (aq) + Cl- (aq) acidic, basic, or neutral? Acid, Base, and Salt Hydrolysis HCl + NH4OH s.a. w.b. NH4Cl (aq) NH4Cl + HOH NH4+ (aq) + Cl- (aq) acidic, basic, or neutral? Acid, Base, and Salt Hydrolysis HCl + NH4OH s.a. w.b. NH4Cl (aq) NH4Cl + HOH NH4+ (aq) + Cl- (aq) Acidic! Will the ions from the salt combine with water? NH4+ + HOH NH4OH + H+ Acid, Base, and Salt Hydrolysis HCl + NH4OH s.a. w.b. NH4Cl (aq) NH4Cl + HOH NH4+ (aq) + Cl- (aq) Acidic! Will the ions from the salt combine with water? NH4+ + HOH NH4OH + H+ w.b. Acid, Base, and Salt Hydrolysis HCl + NH4OH s.a. w.b. NH4Cl (aq) NH4Cl + HOH NH4+ (aq) + Cl- (aq) Acidic! Will the ions from the salt combine with water? NH4+ + HOH NH4OH + w.b. H+ This reaction is OK, since a w.b. is formed Acid, Base, and Salt Hydrolysis HCl + NH4OH s.a. w.b. NH4Cl (aq) NH4Cl + HOH NH4+ (aq) + Cl- (aq) Acidic! Will the ions from the salt combine with water? NH4+ + HOH NH4OH + H+ w.b. Cl- + HOH HCl + OH- This reaction is OK, since a w.b. is formed Acid, Base, and Salt Hydrolysis HCl + NH4OH s.a. w.b. NH4Cl (aq) NH4Cl + HOH NH4+ (aq) + Cl- (aq) Acidic! Will the ions from the salt combine with water? NH4+ + HOH NH4OH + H+ This reaction is OK, since a w.b. is formed w.b. Cl- + HOH HCl (sa) + OH- Acid, Base, and Salt Hydrolysis HCl + NH4OH s.a. w.b. NH4Cl (aq) NH4Cl + HOH NH4+ (aq) + Cl- (aq) Acidic! Will the ions from the salt combine with water? NH4+ + HOH NH4OH + H+ This reaction is OK, since a w.b. is formed w.b. Cl- + HOH H+ + Cl- + OH- Acid, Base, and Salt Hydrolysis HCl + NH4OH s.a. w.b. NH4Cl (aq) NH4Cl + HOH NH4+ (aq) + Cl- (aq) Acidic! Will the ions from the salt combine with water? NH4+ + HOH NH4OH + H+ This reaction is OK, since a w.b. is formed w.b. HOH H+ + OH- Again water cannot make water! NR Acid, Base, and Salt Hydrolysis HCl + NH4OH s.a. w.b. NH4Cl (aq) NH4Cl + HOH NH4+ (aq) + Cl- (aq) Acidic! Will the ions from the salt combine with water? NH4+ + HOH NH4OH + H+ w.b. Cl- + HOH HCl + OHs.a. This reaction is OK, since a w.b. is formed Acid, Base, and Salt Hydrolysis HCl + NH4OH s.a. w.b. NH4Cl (aq) NH4Cl + HOH NH4+ (aq) + Cl- (aq) Acidic! Will the ions from the salt combine with water? NH4+ + HOH NH4OH + H+ This reaction is OK, since a w.b. is formed w.b. Cl- + HOH HCl + OHs.a. Cannot form s.a. from weaker reactants, thus N.R. Acid, Base, and Salt Hydrolysis HCl + NH4OH s.a. w.b. NH4Cl (aq) NH4Cl + HOH NH4+ (aq) + Cl- (aq) Acidic! Will the ions from the salt combine with water? NH4+ + HOH NH4OH + H+ This reaction is OK, since a w.b. is formed w.b. Cl- + HOH HCl + OHs.a. Cannot form s.a. from weaker reactants, thus N.R. Since H+ was formed in the first reaction, then [H+] is now greater than [OH-] making the solution acidic Acid, Base, and Salt Hydrolysis NaCl (aq) Acid, Base, and Salt Hydrolysis NaCl (aq) Na+ (aq) + Cl- (aq) Acid, Base, and Salt Hydrolysis NaCl (aq) Na+ (aq) + Cl- (aq) Acidic, basic, or neutral? Acid, Base, and Salt Hydrolysis HCl + NaOH NaCl (aq) NaCl + HOH Na+ (aq) + Cl- (aq) Acidic, basic, or neutral? Acid, Base, and Salt Hydrolysis HCl + NaOH s.a. NaCl (aq) s.b. NaCl + HOH Na+ (aq) + Cl- (aq) Acidic, basic, or neutral? Acid, Base, and Salt Hydrolysis HCl + NaOH s.a. NaCl (aq) s.b. NaCl + HOH Na+ (aq) + Cl- (aq) Neutral! Acid, Base, and Salt Hydrolysis HCl + NaOH s.a. NaCl (aq) s.b. NaCl + HOH Na+ (aq) + Cl- (aq) Neutral! Now react each of the ions with water. Na+ + HOH NaOH + H+ Acid, Base, and Salt Hydrolysis HCl + NaOH s.a. NaCl (aq) s.b. NaCl + HOH Na+ (aq) + Cl- (aq) Neutral! Now react each of the ions with water. Na+ + HOH NaOH + H+ s.b. Acid, Base, and Salt Hydrolysis HCl + NaOH s.a. NaCl (aq) s.b. NaCl + HOH Na+ (aq) + Cl- (aq) Neutral! Now react each of the ions with water. Na+ + HOH NaOH + s.b. Cannot form strong bases from weaker H+ ones, thus N.R. Acid, Base, and Salt Hydrolysis HCl + NaOH s.a. NaCl (aq) s.b. NaCl + HOH Na+ (aq) + Cl- (aq) Neutral! Now react each of the ions with water. Na+ + HOH NaOH + s.b. Cannot form strong bases from weaker H+ ones, thus N.R. Acid, Base, and Salt Hydrolysis HCl + NaOH s.a. NaCl (aq) s.b. NaCl + HOH Na+ (aq) + Cl- (aq) Neutral! Now react each of the ions with water. Na+ + HOH NaOH + s.b. Cl- + HOH Cannot form strong bases from weaker H+ ones, thus N.R. HCl + OH- Acid, Base, and Salt Hydrolysis HCl + NaOH s.a. NaCl (aq) s.b. NaCl + HOH Na+ (aq) + Cl- (aq) Neutral! Now react each of the ions with water. Na+ + HOH NaOH + s.b. Cl- + HOH Cannot form strong bases from weaker H+ ones, thus N.R. HCl + OHs.a. Acid, Base, and Salt Hydrolysis HCl + NaOH s.a. NaCl (aq) s.b. NaCl + HOH Na+ (aq) + Cl- (aq) Neutral! Now react each of the ions with water. Na+ + HOH NaOH + s.b. Cl- + HOH Cannot form strong bases from weaker H+ ones, thus N.R. HCl + OHs.a. Cannot form strong acids from weaker ones, thus N.R. Buffers Buffers are extremely important in chemistry and biology. They maintain a nearly consistent pH in various solutions. Buffers Buffers are extremely important in chemistry and biology. They maintain a nearly consistent pH in various solutions. Our blood must maintain a pH around 7.35-7.45. If the pH is above 7.45 you would have a condition called alkalosis. If the pH is below 7.35, then one would suffer from acidosis. Buffers Buffers are extremely important in chemistry and biology. They maintain a nearly consistent pH in various solutions. Our blood must maintain a pH around 7.35-7.45. If the pH is above 7.45 you would have a condition called alkalosis. If the pH is below 7.35, then one would suffer from acidosis. Acidosis leads to depression of the nervous system. Mild acidosis can result in dizziness, disorientation, or fainting; a more severe case can cause coma, or death. Buffers Buffers are extremely important in chemistry and biology. They maintain a nearly consistent pH in various solutions. Our blood must maintain a pH around 7.35-7.45. If the pH is above 7.45 you would have a condition called alkalosis. If the pH is below 7.35, then one would suffer from acidosis. Acidosis leads to depression of the nervous system. Mild acidosis can result in dizziness, disorientation, or fainting; a more severe case can cause coma, or death. What would happen to the pH of our blood if we were to eat acidic foods, such as apples, oranges, or limes? What might happen to the pH of our blood if some of the hydrochloric acid from our stomach were to seep into our blood? Buffers Buffers are extremely important in chemistry and biology. They maintain a nearly consistent pH in various solutions. Our blood must maintain a pH around 7.35-7.45. If the pH is above 7.45 you would have a condition called alkalosis. If the pH is below 7.35, then one would suffer from acidosis. Acidosis leads to depression of the nervous system. Mild acidosis can result in dizziness, disorientation, or fainting; a more severe case can cause coma, or death. What would happen to the pH of our blood if we were to eat acidic foods, such as apples, oranges, or limes? What might happen to the pH of our blood if some of the hydrochloric acid from our stomach were to seep into our blood? The pH would be lower in both Despite the possibility of pH increases or decreases, the body maintains a nearly constant pH of 7.4. The body uses buffers to maintain this remarkable feat. What is a buffer and how does it work? Despite the possibility of pH increases or decreases, the body maintains a nearly constant pH of 7.4. The body uses buffers to maintain this remarkable feat. What is a buffer and how does it work? A buffer consists of a weak acid and the salt of its conjugate base, or a weak base and the salt of its conjugate acid. Examples: HF + NaOH w.a. NaF + HOH c.b. Despite the possibility of pH increases or decreases, the body maintains a nearly constant pH of 7.4. The body uses buffers to maintain this remarkable feat. What is a buffer and how does it work? A buffer consists of a weak acid and the salt of its conjugate base, or a weak base and the salt of its conjugate acid. Examples: HF + NaOH w.a. NH3 + HCl w.b. NaF + HOH c.b. NH4Cl c.a. HF (g) Buffer preparation: Add 0.10 mole HF (g) and NaF (s) to 1.0 L of water. 1.0 L NaF (s) HF (g) Buffer preparation: Add 0.10 mole HF (g) and NaF (s) to 1.0 L of water. HF (g) large NaF (s) H + + Fsmall Na+ + F- 1.0 L NaF (s) H+ Na+ HF F- HCl Buffer preparation: Add 0.10 mole HF (g) and NaF (s) to 1.0 L of water. HF (g) large NaF (s) H + + Fsmall Na+ + F- Now add the strong acid HCl HF Na+ H+ 1.0 L F- HCl Buffer preparation: Add 0.10 mole HF (g) and NaF (s) to 1.0 L of water. HF (g) Large NaF (s) H + + F- small Na+ + F- HF Na+ H+ 1.0 L H+ F- Cl- Now add the strong acid HCl HCl H+ + Cl- What will the pH be if just water and no buffer? HCl Buffer preparation: Add 0.10 mole HF (g) and NaF (s) to 1.0 L of water. HF (g) H+ + FLarge NaF (s) small Na+ + F- H+ Na+ HF 1.0 L H+ F- Cl- Now add the strong acid HCl HCl H+ + Cl- What will the pH be if just water and no buffer? pH = 1, dead if this is your blood. HCl Buffer preparation: Add 0.10 mole HF (g) and NaF (s) to 1.0 L of water. HF (g) H+ + FLarge NaF (s) small Na+ + F- H+ Na+ HF 1.0 L H+ F- Cl- Now add the strong acid HCl HCl H+ + Cl- What will the pH be if just water and no buffer? pH = 1, dead if this is your blood. What removes the H+ to keep the pH near 7? HCl Buffer preparation: Add 0.10 mole HF (g) and NaF (s) to 1.0 L of water. HF (g) H+ + Fsmall Large Na+ + F- NaF (s) H+ Na+ HF 1.0 L H+ F- Cl- Now add the strong acid HCl HCl H+ + Cl- What will the pH be if just water and no buffer? pH = 1, dead if this is your blood. What removes the H+ to keep the pH near 7? The conjugate base, FH + + F- HF (a weak acid, low H+ ) NaOH Buffer preparation: Add 0.10 mole HF (g) and NaF (s) to 1.0 L of water. HF (g) H+ + FLarge NaF (s) small Na+ + F- H+ Na+ HF 1.0 L Na+ F- OH- Now add the strong base NaOH What will the pH be if just water NaOH Na+ + OHand no buffer? NaOH Buffer preparation: Add 0.10 mole HF (g) and NaF (s) to 1.0 L of water. HF (g) H+ + FLarge NaF (s) small H+ Na+ HF 1.0 L Na+ + F- Na+ F- OH- Now add the strong base NaOH What will the pH be if just water and NaOH Na+ + OHno buffer? PH = 13, dead again What removes the OH- to keep the pH near 7? The acid HF HF + OH- F- + HOH Titration Titration is an experimental procedure to determine the concentration of an unknown acid or base. The figure on the left shows the glassware for a titration experiment. A buret clamp holds the buret to a ring stand and below the buret is a flask containing the solution to be titrated, which includes an indicator. The purpose of the indicator is to indicate the point of neutralization by a color change. NaOH + HCl NaCl + HOH The picture on the left shows the tip of a buret, with air bubble, which is not good, and also shows the stop-cock. Note the position of the stop-cock is in the “off” position. This picture shows the color of the phenolphthalein indicator at the end-point. In this experiment a 23.00 mL aliquot of 0.1000 M NaOH titrant is added to 5.00 mL of an unknown HCL solution. The acid solution in the beaker starts out clear and becomes pink when all of the HCL has been consumed. Titration How can we calculate the concentration of acid in the beaker? Titration How can we calculate the concentration of acid in the beaker? Normal procedure, yes, a conversion. Steps 1-4, again! How can we calculate the concentration of acid in the beaker? Normal procedure, yes, a conversion. Steps 1-4, again! 0.100 mole NaOH L NaOH solution How can we calculate the concentration of acid in the beaker? Normal procedure, yes, a conversion. Steps 1-4, again! 0.100 mole NaOH 10-3 L solution mL solution L NaOH solution How can we calculate the concentration of acid in the beaker? Normal procedure, yes, a conversion. Steps 1-4, again! 0.100 mole NaOH L NaOH solution 10-3 L solution 23.00 mL soln mL solution How can we calculate the concentration of acid in the beaker? Normal procedure, yes, a conversion. Steps 1-4, again! 0.100 mole NaOH L NaOH solution 10-3 L solution 23.00 mL soln mole HCl mole NaOH mL solution How can we calculate the concentration of acid in the beaker? Normal procedure, yes, a conversion. Steps 1-4, again! 0.100 mole NaOH L NaOH solution 10-3 L solution 23.00 mL soln mole HCl mole NaOH mL solution How can we calculate the concentration of acid in the beaker? Normal procedure, yes, a conversion. Steps 1-4, again! mL HCl soln. 0.100 mole NaOH 10-3 L solution 23.00 mL soln mole HCl mole NaOH 10-3 L HCl soln. L NaOH solution mL solution How can we calculate the concentration of acid in the beaker? Normal procedure, yes, a conversion. Steps 1-4, again! 0.100 mole NaOH 10-3 L solution 23.00 mL soln mole HCl L NaOH solution mL solution mL HCl soln. mole NaOH 10-3 L HCl soln. 5.00 mL How can we calculate the concentration of acid in the beaker? Normal procedure, yes, a conversion. Steps 1-4, again! 0.100 mole NaOH 10-3 L solution 23.00 mL soln mole HCl mL HCl soln. mole NaOH 10-3 L HCl soln. L NaOH solution mL solution 5.00 mL = 0.460 M HCl Indicators Indicators are weak organic (carbon containing) acids of various colors depending on the formula of the acid. Below is a generic acid. HA H+ + Acolorless 1. pink Describe the color change when a strong acid is added? Indicators Indicators are weak organic (carbon containing) acids of various colors depending on the formula of the acid. Below is a generic acid. HA H+ + Acolorless 1. Less pink pink Describe the color change when a strong acid is added? Indicators Indicators are weak organic (carbon containing) acids of various colors depending on the formula of the acid. Below is a generic acid. HA H+ + Acolorless pink 1. Describe the color change when a strong acid is added? Less pink 2. Describe the color change when a strong base is added? Indicators Indicators are weak organic (carbon containing) acids of various colors depending on the formula of the acid. Below is a generic acid. HA H+ + Acolorless pink 1. Describe the color change when a strong acid is added? Less pink 2. Describe the color change when a strong base is added? Darker pink Indicators Indicators are weak organic (carbon containing) acids of various colors depending on the formula of the acid. Below is a generic acid. HA H+ + A- colorless pink 1. Describe the color change when a strong acid is added? Less pink 2. Describe the color change when a strong base is added? Darker pink 3. Describe the color change when the pH is lowered? Indicators Indicators are weak organic (carbon containing) acids of various colors depending on the formula of the acid. Below is a generic acid. HA H+ + Acolorless pink 1. Describe the color change when a strong acid is added? Less pink 2. Describe the color change when a strong base is added? Darker pink 3. Describe the color change when the pH is lowered? Less pink Indicators Indicators are weak organic (carbon containing) acids of various colors depending on the formula of the acid. Below is a generic acid. HA H+ + Acolorless pink 1. Describe the color change when a strong acid is added? Less pink 2. Describe the color change when a strong base is added? Darker pink 3. Describe the color change when the pH is lowered? Less pink 4. Describe the color change when the pH is raised? Indicators Indicators are weak organic (carbon containing) acids of various colors depending on the formula of the acid. Below is a generic acid. HA H+ + Acolorless pink 1. Describe the color change when a strong acid is added? Less pink 2. Describe the color change when a strong base is added? Darker pink 3. Describe the color change when the pH is lowered? Less pink 4. Describe the color change when the pH is raised? Darker pink Color versus pH of Many Different indicators How can we make an indicator? How can we make an indicator? Step One Red Cabbage Step Two Cook the Cabbage Step Three Filter the Juice What color is the juice after filtering? What color is the juice after filtering? The color of pH 6, 7, or 8 Colors of cabbage juice at various pH values The End Ch#14 ACIDS BASES AND SALTS





![pH = - log [H + ]](http://s2.studylib.net/store/data/005622524_1-002df1ea50d2a849b15deb604928664e-300x300.png)

