ppt - Erice Crystallography 2004
advertisement
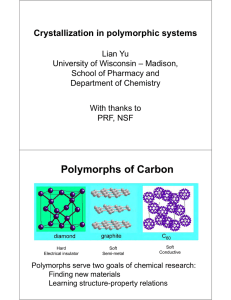
Polymorphism and Structure-Property Relationships Diversity amidst Similarity 35th Crystallography Course Erice, Italy July 9 - 20, 2004 Michael D. Ward Department of Chemical Engineering and Materials Science and University of Minnesota Materials Research Science and Engineering Center University of Minnesota - Minneapolis “Classical Materials” • Structural materials • Electrical conductors • Ceramics • Computer chips • Lasers Stretto di Messina amorphous metals ceramic superconductor Intel microchip • Design and Synthesis • Processing • Characterization ferrite core memory • Applications Polymorphism in “conventional materials” Engineering properties through control of crystal phase structural steels (“tough”) hard steels (brittle) Polymorphism in conventional materials Quartz • • • • • Quartz oscillators Frequency control Mass measurement Sensors Alpha form required Polymorphism in conventional materials Carbon Hard Electrical insulator Thermal conductor Soft Semi-metal Lubricant Soft Conductive Exotic Molecular Crystal! Properties in Molecular Materials Metallic conductivity Superconductivity Piezoelectricity Pyroelectricity Non-linear optical activity Second harmonic generation Ferroelectricity Semiconductivity Light emission Photovoltaicity Photoconductivity Color Bioactivity Molecular Materials: Technologies • Xerography • Displays • Pigments • Pharmaceuticals • Light-emitting diodes • Flexible electronics Attributes of Molecular Materials • Low-cost processing • Molecular design = control of properties • Properties depend on solid state structure ---------• Challenge: Control of solid state structure • Polymorphism • Structure-property relationships only partially developed • Survey structures • Find true causal relationships Structure-property relationships in polymorphs different properties Identify properties that DO depend on crystal structure (specific) polymorphs same properties Identify properties that DO NOT depend on crystal structure (not so specific) Polymorphism and Reactivity Schmidt, G. M. J. Organic conductors • First discovered early 1960s; TCNQ salts “A new class of organic solids has recently been prepared which exhibits properties unusual for organic systems” [Chestnut and Arthur, J. Chem. Phys. 1962, 36, 2969] • Exhaustive investigations: crystal engineering • Targeted properties - Semiconductors - Metals - Superconductors - Magnets S S S S TTF • TTF-TCNQ: metallic (TTF0.59+TCNQ0.59-) NC CN • Properties depend on solid-state structure NC CN TCNQ Why examine polymorphs? S S S S NC NC CN CN NC NC CN CN F NC F CN NC CN NC CN NC CN F F • TTF-TCNQ: uniform stacks; bond-over-ring; metal • TTF-TCNQF2: dimerized stacks; ring-over-ring; semiconductor • Difficult to separate effect of structure from effect of substituents Blessing, Coppens Kistenmacher, et al. TTF-TCNQ polymorphism S S S S NC CN NC CN Peierls transition Peierl’s Transition increasing temperature Cowan, et al. metal semiconductor High temp P21/c Low temp P21/c Organic metals Conductivity • Interplanar separation • Uniform spacing • Ring overlap • Electronic configuration (band filling) D+o + S S S S S S b 4t Do+ S S Peierls gap (semiconductor) BEDT-TTF (ET) S S S S S S S 2 S Solution with ET and X- - e- [ S S S S S S S S ] • Often made by electrocrystallization • Extensive variety of counterions (X-) • Polymorphs common • Metallic, superconducting • ET2I3 : 14 polymorphs reported • Distinguished by electrical properties (?) • Length of X-X-X- and Y-X-Y- anions affect interplanar spacing • Internal lattice pressure suppresses Peierls transition • Increase Tc Williams, et al. + 2 ET2I3 Polymorphs • b-form: thermodynamically preferred S S S S • a-form, P-1, metal-semiconductor transition • b-form, P-1, superconducting at 1.4 K S S S S • k-form, P21/c, superconducting at 3.6 K • Selectivity controlled by growth rate, epitaxy Williams, et al. Substrate directed polymorph control b1 b2 10 nm Monolayer growth Coincident epitaxy No epitaxy for a-phase ß-(ET)2I3 (001) on graphite (no a polymorph) Last, et al. TMTSeF-TCNQ (dimorphic) segregated stack (metal) H3C Se Se CH3 H3C Se Se CH3 NC CN NC CN mixed stack (semiconductor) mixed stacks (semiconductor) Bechgaard, Kistenmacher, Cowan Mixed Stack Complexes D+ AD+ AD+ A- D A D A D A neutral D2+ A2D2+ A2D2+ A2ionic doubly ionic Barochromic polymorphs S S Cool to 81 K or apply pressure TTF S Cl S Cl DA P21/n; yellow O O Cl chloranil <e2/r> ! D+APn; red; ferroelectric smaller D…A spacing Cl Torrance, et al. Molecular magnets ferromagnetic c = 1/(T – q) M = cH thru-space spin exchange c paramagnetic O antiferromagnetic M M 1/T paramagnetism Ferromagnetism • • • • • • • Spin moments tend to align parallel Permanent moment (in absence of H) FM < Tc < paramagnet FM domains grow in presence of field Cooperative interactions in solid state Complicated, poorly understood Necessary, but not sufficient conditions identified H ferromagnetism antiferromagnetism ferrimagnetism H Molecular magnets NC Fe + D+ANC decamethylferrocene Purple crystals Paramagnetic DMFc+; S = ½ Antiferromagnetic (TCNQ)22- Miller, et al. CN CN TCNQ Green crystals Staggered Cp* rings Metamagnetic dFe…Fe = 9.635 Å dA…A = 3.478 Å (AF) Purple crystals Eclipsed Cp* rings Ferromagnetic dFe…Fe = 8.609 Å (F) dA…A = 3.635 Å Metamagnetism in DMFc+-TCNQ- NC Fe + decamethylferrocene CN D+ANC CN TCNQ Hydrogen-bonded magnets H3C CH3 -O OH CH3 +N N . CH3 O HQNN OH a a-form • intramolecular and intermolecular H-bonds • 3-D ferromagnet b b-form • intermolecular H-bonds • p-p stacking • ferromagnet with weak AF at low T Matsushita, et al. Hydrogen-bonded magnets (a-HQNN) hyperconjugation bifurcated H-bond dimer OH…O H-bond positive CH…ON contact negative • Odd alternant hydrocarbons (McConnell) • Spin distribution alternates sign and magnitude at each carbon atom • Overlap favored for carbon atoms have opposite spin densities Magnetism and polymorphism F S S 3.4 Å S…N F N CN N F F 3.1 Å S…N Conformational polymorphs (slight) a-form P-1 Antiferromagnetic Anti-parallel head-to-tail chains 3.0 Å S…N 3.67 Å S…S (AF) b-form Fdd2 Ferromagnetic, Tc = 35.5 K Parallel head-to-tail chains Banister, et al. Polar crystals Polar crystals from acentric molecules Grand challenge in organic solid state chemistry Hydrogen bonded aggregates Metal coordination networks Antiparallel ionic sheets Head-to-tail alignment of dipolar guests Limited de novo design Properties Piezoelectricity Pyroelectricity Ferroelectricity Second harmonic generation -------Structure-property relationships complex (polar axis, phase matching, absorption…) • Perhydrotriphenylene • Thiourea Polar crystals Form 1 N NO2 PAN H3COHCHN • Both crystallize in polar space group P21 • Form 1 grows under slow growth conditions; not SHG-active • Form 2 grows under rapid growth conditions; SHG active • Same trend for FBNH • Thermodynamically more stable “non-polar” forms favored under slow growth NO2 O N O H Form 2 H FBNH Aldoshin, et al. Polymorphism and Transistors • Campbell & Trotter, Acta Cryst, 1962, 15, 289 • Minakata, et al., J. Appl. Phys. 1992, 72, 5220 +++++++ -------- • Bouchoms, et al. Synth. Met. 1999, 104, 175 • Holmes, et al., Chem. Eur. 1999, 5, 3399 • Siegrist, et al., Angew. Chem. Int. Ed. Engl. 2001, 40, 1732 (Bell labs) • Mattheus, et al., Synth. Met. 2003, 138, 475 • Fritz, et al.. J. Am. Chem. Soc., 2004, 126, 4084 • Pentacene films of technological interest • Confusion about bulk polymorphs • Only one bulk polymorph unambiguously identified • Thin-film phases: 15.4 Å, 15.0 Å, 14.4 Å, 14.1 Å (bulk) • Thickness and substrate affects “polymorph” • Structure affects transport • Transport in layers near the gate electrode important Pentacene monolayer (AFM) Grazing incidence X-ray diffraction SSRL (M. Toney) Bulk pentacene a = 6.266 Å, b = 7.775 Å, and g = 84.684º Monolayer/Thin film a = 5.916 Å, b = 7.588 Å, and g = 89.95º Polymorphism depends on thickness Fritz, et al. Kidney stones: A major health issue COM - 98% (protein - 2%) Kidney stone formation nucleation – growth – aggregation - attachment to cells Stages of stone formation • Calcium oxalate monohydrate (COM) aggregates and adheres to epithelial cells • Calcium oxalate dihydrate (COD) “protective” • Calcium oxalate trihydrate (COT) rare WHY? • Crystal attachment/aggregation influenced by urinary macromolecules • Knockout mice show COM attachment to epithelial cells Summary • Polymorphs provide an opportunity to isolate solid state structure from other factors (e.g., substituents) • Requires in-depth analysis of properties that are not affected by solid state structure (less obvious) as well as properties that are affected (not obvious) • Some structure-property relationships apparent (e.g., crystal polarity, segregated vs. mixed stack) • Others not so apparent (e.g., ferromagnetism) • How many structures required? Shark attacks • Does correlation imply causation? Ice cream sales True then, true today “…the synthetic chemist is likely to have many opportunities to encounter unusual phenemona by accident during everyday chemical work with crystalline solids and without the proper background will not be prepared to recognize and take advantage of such chance discoveries. There is the further misfortune that those workers in areas where the most dramatic applications of polar materials have occurred are, in general, uncomfortable when dealing with structures of complex organic molecules; the result is a serious lack of communication between groups whose interaction should be mutually beneficial.” -Curtin and Paul, Chemical Reviews, 1981