Chapter 13 - Cell Metabolism
advertisement

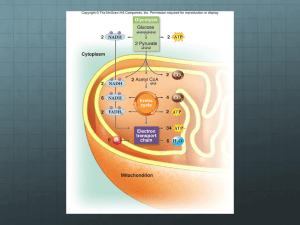
Chapter 13 How Cells Obtain Energy from Food From Chapter 3 (Energy) • Sun is source of all energy • Through photosynthesis/dark reactions, plants convert solar energy chemical energy + sugars • Other organisms consume sugars, convert sugars to chemical energy – Chemical bond energy in food – Catabolism of sugars (glucose) is most direct pathway to chemical energy Sugar Chemical Energy • Use steps to harvest all the energy and not waste it as heat • Aerobic metabolism yields the most energy (O2 needed) • Metabolites more oxidized than glucose – Enzymes catalyze reactions • Oxidation reactions must be coupled with reduction reactions – The reduced molecules are the carriers (NADH and NADPH) Sugar Chemical Energy • Overall products of sugar catabolism: – CO2 – H2O – Reduced (activated) carriers • NADH • NADPH – In mitochondria, reduced carriers now oxidized (lose electrons) – Electrons released to electron transport system » Allow ATP synthesis in mitochondria Stage 1 - Digestion In eukaryotes (mammals): • Digestion – HCl -- stomach – Enzymes – mouth, stomach, small intestine – Enzymes in the lysosome for internal cellular digestion • Absorption through specialized cells in small intestine bloodstream body’s cells • Metabolism begins in cell cytosol Location of Macromolecules in Cell Stage 2 - Glycolysis • Glycolysis starts in the cytoplasm • Glucose (6C) 2 pyruvate (3C each) – Other sugars can be used but must convert to intermediates of glycolysis • 2 carrier molecules generated per pyruvate – 2 molecules ATP (carries energy) – 2 reduced NADH (carries electrons) • Pyruvate molecules move to the mitochondria Stage 3 – Kreb’s Cycle/ETC • In the mitochondria pyruvate broken down to CO2 and the remaining 2 Cs (acetyl group) are added to Coenzyme A – Also can get Acetyl CoA from fats • Each acetyl CoA transfers the 2C’s to citric acid cycle where carrier molecules are generated – GTP carries energy – NADH/FADH2 carry electrons • Electrons electron transport chain – Release energy used for oxidative phosphorylation – O2 needed for successful reaction ADP + Pi ATP – ATP moved to the cytosol for use Glycolysis • 10 reactions, each catalyzed by an enzyme • Products or intermediates become more oxidized through pathway – Doesn’t react with oxygen atoms; rather lose electrons to carriers • 2 NADH generated from catabolism 1 glucose • Some steps are not spontaneous (+DG) – Coupled with subsequent spontaneous reactions Glycolysis • Uses 2 ATP to catabolize glucose – In coupled reactions – hydrolysis of ATP allows nonspontaneous reactions to proceed – Phosphates from ATP added to intermediates • Form high energy phosphate bonds • Now intermediates have higher energy • In later steps, generates 4 ATP – When phosphates cleaved from intermediates • Overall glycolysis yields (net gain) 2 ATP Overall Process Glycolysis Glycolysis Glycolysis Glycolysis Glycolysis Net Result of Glycolysis • Glucose + 2 ATP 2 NADH + 4 ATP + 2 pyruvate • Net energy outcome 2 NADH and 2 ATP What to Know About Glycolysis • 10 enzymes / 5 reaction types – Kinases – add a phosphate group to intermediates, phosphate transfer – Isomerases – rearranges the atoms in the intermediates – Dehydrogenase – causes a redox reaction, electron ends up on FADH2 or NADH – Dehydrations – removal of H2O – Cleavage reaction – split glucose to 2 3-C molecules • Net outcome of glycolysis Steps and Reactions • • • • • • • • • • Step 1 – kinase, phosphate transfer Step 2 – isomerase, rearrange atoms Step 3 – kinase, phosphate transfer Step 4 – cleavage to 2 3-C molecules Step 5 – isomerase, rearrange atoms Step 6 – dehydrogenase, make NADH Step 7 – kinase, phosphate transfer Step 8 – isomerase, rearrange atoms Step 9 – removal of H2O Step 10 – kinase, phosphate transfer Enzymatic Coupling • Steps 6 and 7 are coupled to take advantage of the highenergy phosphate intermediate to create ATP • Step 6: glyceraldehyde 3-phosphate has a inorganic phosphate group added to create 3-phosphoglycerate, substrate for Step 7 and generates NADH • Step 7: 1,3-bisphosphoglycerate releases the energy in the phosphate bond to create 1 ATP for each 1,3bisphosphoglycerate Overall Results • Enzyme-mediated energy storage through coupled reactions to create high energy bonds • Intermediate is higher in energy than before – Has second high energy phosphate bond – NADH is generated and will also increase energy when it participates in oxidative phosphorylation High Energy Bonds Fermentation • Can generate ATP in absence of O2 – anaerobic • Anaerobic organisms create ATP through glycolysis – Pyruvate converted to ethanol and CO2 (yeast) or lactate (muscle) • Process called fermentation Stage 3 • Pyruvate is moved to the mitochondria • In the presence of O2 it is converted to 1 molecule of CO2 and the remaining 2 C’s are attached to Coenzyme A, creating Acetyl CoA using pyruvate dehydrogenase complex • Also generates a molecule of NADH Fatty Acids as Energy Source • Fatty acids can be linked to CoA (fatty acyl CoA) and therefore yield acetyl CoA that can enter the citric acid cycle • Generates NADH and FADH2 for each acetylCoA • Amino acids also can be made to acetylCoA and used in the Kreb’s cycle Energy Produced in Mitochondria • Fats and sugars are major sources of energy • Acetyl CoA is made in the mitochondria • No surprise to learn that the energy is also harvested in the mitochondria • In bacteria – glycolysis and citric acid cycle in cytosol Citric Acid Cycle • 2/3 of oxidation of C compounds in the average cell • End product is CO2 (waste) and NADH high energy molecules (used later) • Requires O2 to regenerate NAD+ but not actually used in reactions • Link the acetyl group of Acetyl CoA to 4 C molecule, oxaloacetate, to make 6 C citrate • By end of cycle, all the C of glucose is released as CO2, remembering that 1 CO2 was released in previous stage Citric Acid Cycle (TCA Cycle, Kreb’s Cycle) *** Activated Carriers Two new energy molecules are introduced – FADH2 (flavin adenine dinucleotide) • High energy electrons and H – GTP (ribonucleotide) • Similar to ATP and will give up PO4 to ADP to make ATP • Requires O2 but as H2O (red circle) • Some of the steps products can leave mitochondria and used in the cytosol to make precursors like amino acids Steps and Reactions • Step 1 – add acetyl CoA to oxaloacetate, citrate (6 C) • Step 2 – isomerase, rearrange atoms (6 C) • Step 3 – dehydrogenase, make NADH, lose CO2 (5 C) • Step 4 – dehydrogenase, make NADH, lose CO2, add CoA back to molecule (4 C) • Step 5 – generate GTP, remove CoA (4 C) • Step 6 – dehydrogenase, make FADH2, rearrange atoms (4 C) • Step 7 – add H2O (4 C) • Step 8 – dehydrogenase, make NADH, regenerates oxaloacetate (4 C), why a cycle Electron-Transport Chain • Final step in energy generation – most energy released here • e- of NADH and FADH2 move through the chain, moving to lower energy level • Occurs in the inner membrane of the mitochondria • Specialized molecules accept and donate e- as they move down chain • Create an electrochemical gradient – As e- move down chain, H+ move across the membrane, altering the concentration of H+ on either side = gradient – Gradient used to generate ATP (Chapter 14) Oxidative Phosphorylation • e- eventually end up on O2 and with the H+ form H2O – e- is at least energy level • Complete oxidation of glucose produces 6 CO2, H2O and ~30 ATP • Glycolysis alone produces just 2 ATP • In bacteria – plasma membrane • In eukaryotes – in the inner mitochondrial membrane Storing and Using Food • Need to generate ATP constantly, can because store “food” within our cells • Fatty acids in fat cells, globules in cells – Holds more energy gram for gram than sugar • Glucose stored as glycogen, a branched polysaccharide in granules in animal cell cytoplasm – Used when not enough glucose in bloodstream – Released as glucose 1-phosphate and can enter glycolysis Sugar Storage in Plants and Mammals Plants • Have chloroplasts as well as mitochondria – Mitochondria will generate ATP from the sugars made during photosynthesis • Especially in cells without chloroplasts such as roots or when without sunlight • Excess sugars can be converted to fats or starch, the equivalent to glycogen in animals, different branching pattern – Stored in the chloroplast Chloroplasts and Mitochondria • Chloroplasts make ATP and NADPH that cannot leave • ATP and NADPH converted to sugar that can leave and be used in glycolysis and ATP generation in the mitochondria and into other building blocks Biosynthetic Pathways Begin with Glycolysis or TCA Cycle • Intermediates can be used by other enzymes as the starting point in making amino acids, nucleotides, lipids and other small organic compounds • Black arrows – one enzyme reaction • Red arrows – multi step reactions Pathway Interactions • Some molecule can be substrate in many different pathways • Elaborate network of control mechanisms Bringing It All Together Metabolism High ATP Levels Low ATP Levels Anabolism Catabolism Glycogen Fats Proteins Glycogen Fats Proteins