Control of Microorganisms: Sterilization & Disinfection
advertisement
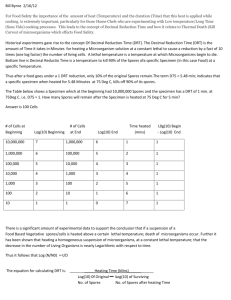
Control of Microorganisms Bio3124 Lecture #6 Definitions sterilization destruction /removal of all viable organisms disinfection killing, inhibition, removal of pathogens disinfectants usually chemical used on inanimate objects sanitization reduction of microbial population to levels deemed safe antisepsis prevention of infection of living tissue by microorganisms antiseptics chemical agents, kill or inhibit growth of microorganisms when applied to tissues Chemotherapy: internal use chemicals to kill or inhibit microbes within host tissues Definitions… Antimicrobials: -cidal agents: agent kills, commonly called germicides kills pathogens and many nonpathogens but not necessarily endospores include bactericides, fungicides, algicides, and virucides -static agents: agent inhibits growth include bacteriostatic and fungistatic Microbial Death microorganisms are not Bacterial Death Curve killed instantly 120 Plot: log of survivors vs antimicrobial exposure time The slope: average death rate 100 1E+09 1E+08 80 1E+07 1E+06 60 100000 10000 40 1000 100 20 10 0 1 1 2 3 4 5 6 7 Time 8 9 10 11 12 Survivors( log of CFU/ml) exponential 1E+10 Survivorsx109 (CFU/ml) death curves are 1E+11 Effectiveness of Antimicrobial Agent Activity Depends on: population size population composition vegetative vs dormant concentration duration of exposure longer exposure more organisms killed temperature higher temperatures usually increase killing local environment e.g., pH, viscosity and concentration of organic matter organisms in biofilms are physiologically altered and less susceptible to many antimicrobial agents Methods in controlling microorganisms Two major methods are used, Physical methods Heat Moist heat sterilization (autoclaves) Pasteurization Dry heat sterilization (ovens, incinerators) Low temperature (refrigeration, freezing) Filtration (for heat labile liquids) Irradiation (UV and ionizing radiation) Chemical methods Disinfectants and antiseptics (phenolics,alcohols, aldehydes, gases… etc) Chemotherapeutic agents (internal use) Moist Heat Sterilization above 100oC , requires saturated steam under pressure (autoclave) effective against all types of microorganisms and spores degrades nucleic acids, denatures proteins, and disrupts membranes The Autoclave or Steam Sterilizer Autoclave 121°C, 15 psi (2 atm) for 20 minutes Kills all bacteria Kills endospores Clostridium botulinum Botulism Bacillus anthracis – Anthrax Pasteurization Louis Pasteur and Claude Bernard (1862) does not sterilize logarithmic reduction of germs rather than killing them all Most often ~5 log reduction; milk, beer, apple cider, fruit juice and other beverages Procedures High temperature short time: holding milk at 72 C for 15-30 seconds Ultra high temperature: exposure to ~130 C for a fraction of second Double pasteurization: 68C for 30 minutes followed by cooling and again heating at 68C for additional 30 minutes (spores germinate, killed upon entry to vegetative stage) Dry Heat Sterilization less effective Clostridium botulinium spores killed in 2-3 hours Ovens: higher temperatures & longer exposure time (160-170oC for 2 to 3 hours) oxidizes cell constituents and denatures proteins Bench-top incinerators inoculating loops Institutional incineration Measuring Heat-Killing Efficiency To develop standards for killing efficiency: specially important for industrial settings to develop SOPs decimal reduction time (D or D value) time required to kill 90% of microorganisms or spores in a sample at a specific temperature One log reduction Kinetics of thermal reduction 106 D is the time required for one log reduction (90% kill) Can be calculated using: Δt # Bacteria DT= 105 100oC 1 log 104 log N1-logN2 Δt: total exposure time N1: initial population N2: population size after treatment T= applied Temperature D100 103 Time Example 1: calculate the D value for a bacterial suspension of 109 cfu/ml that was subjected to 85˚C for 15 minutes at which point its density was reduced to 106 cfu/ml. Δt DT= log N1-logN2 15 D85= log 109-log106 15 D85= 9- 6 D85= 5 minutes Δt: 15 minutes N1: 109 cfu/ml N2: 106 cfu/ml T= 85˚ C Example 2: the D90 value for a bacterium is 2 minutes. If starting culture has 108 cfu/ml, how long should this suspension be kept at 90C to kill the entire population? Δt DT= log N1-logN2 Δt 2= log 108-log100 Δt 2= 8- 0 Δt = 16 minutes Δt: ? minutes N1: 108 cfu/ml N2: 1 cfu/ml T= 90˚ C The D value: an index for sensitivity to thermal killing 106 • Which one is more sensitive to heat killing at 100˚C? Bacillus subtilis or E.coli? # Bacteria • At 100C the time required to reduce Bacillus population is longer than that required for E.coli 105 104 DE.coli 103 DB.subtilis 100oC Time The D value is temperature dependent # Bacteria 106 D value decreases as the temperature increases ie. there is less time required to reduce the population by one log at higher temperatures 105 104 D120 120oC D110 D100 110oC 103 Time 100oC Kinetics of thermal reduction: the Z value Z value 100 increase in temperature required to reduce D value (min) D by 1/10 (one log reduction) ΔT Z= 10 ΔT: Temperature change D1: initial D value D2: secondary D value 1 log 1 Z =10˚C 100 105 log D1-logD2 110 115 120 Temperature (T) Kinetics of thermal reduction: the Z value by having D values for different temperatures one can seek for altering the sterilization protocol to fit to the industrial setting One question would be: how much the temperature can increase to reduce the D value to a given length This would provide a pragmatic approach in setting up SOPs in industrial settings The use of Z value Example: A food processing company produces canned meat. Prevention of Clostridium botulinum spores from growing is important. The D121 for botulinum spores is 0.2 minutes and the Z value is 10˚C. the company wants to sterilize the canned food at 111˚C. what should be the length of sterilization if they consider to kill 1012 spores per can content. since every 10˚C decrease in treatment causes 10-fold increase in D value then: D111= D121x10 ie. D111= 0.2x10 = 2 minutes using, Δt D111= log1012-log100 Δt 2= 12- 0 Δt= 2x12= 24 minutes They should heat treat their product at 111˚C for 24 minutes. Problem : try this on your own The Z value for a microorganism is 2oC. it takes 54 minutes at 75oC to reduce the population from 109 to 106. At what temperature should the microorganism be treated to achieve the same result in 10.8 sec. Answer=800C Low Temperatures Freezing stops microbial reproduction due to lack of liquid water some microorganisms killed by ice crystal disruption of cell membranes Refrigeration slows microbial growth and reproduction Does not prevent psychrophilic microorganisms Filtration Porous material with 0.1-0.45 um pore size reduces microbial population or sterilizes solutions of heatsensitive materials by removing microorganisms also used to reduce microbial populations in air Filtration Bacillus megaterium Trapped on a nylon Membrane with 0.2 um pore size Enterococcus faecalis Trapped on a polycarbonate Membrane with 0.4 um pore size Filtering air surgical masks cotton plugs on culture vessels high-efficiency particulate air (HEPA) filters used in laminar flow biological safety cabinets Ultraviolet (UV) Radiation limited to surface sterilization because it does not penetrate glass, dirt films, water, and other substances has been used for water treatment Kills by inducing massive number of mutations How about escaping mutants? Ionizing Radiation Gamma radiation from cobalt 60 is used penetrates deep into objects destroys bacterial endospores; not always effective against viruses used for sterilization of antibiotics, hormones, sutures, plastic disposable supplies, and food Chemical Control Agents -Disinfectants and Antiseptics Phenolics commonly used as laboratory and hospital disinfectants (2%) act by denaturing proteins and disrupting cell membranes tuberculocidal, effective in presence of organic material, and long lasting disagreeable odor and can cause skin irritation Alcohols bactericidal, fungicidal, but not sporicidal Effective if diluted to 70% in water (95% is much less active) inactivate some enveloped viruses denature proteins and possibly dissolve membrane lipids Halogens - Iodine skin antiseptic oxidizes cell constituents and iodinates proteins at high concentrations may kill spores skin damage, staining, and allergies can be a problem Halogens - Chlorine oxidizes cell constituents important in disinfection of water supplies and swimming pools, used in dairy and food industries, effective household disinfectant destroys vegetative bacteria and fungi, but not spores can react with organic matter to form carcinogenic compounds Quaternary Ammonium Compounds detergents that have antimicrobial activity and are effective disinfectants organic molecules with hydrophilic and hydrophobic ends cationic detergents are effective disinfectants kill most bacteria, but not Mycobacterium tuberculosis or endospores safe and easy to use, but inactivated by hard water and soap Aldehydes highly reactive molecules that cross link proteins sporicidal and can be used as chemical sterilants combine with and inactivate nucleic acids and proteins Sterilizing Gases used to sterilize heat-sensitive materials EtO penetrates plastic packages Toxic, needs to be aerated microbicidal and sporicidal combine with and inactivate proteins BPL used for sterilizing vaccines Decomposes after use, but is carcinogenic Evaluation of antimicrobial efficiency: Phenol coefficient 5 Minutes 5 Minutes TEST Phenol 10 Minutes 10 Minutes Evaluation of antimicrobial efficiency calculation: The reciprocal of the lowest concentration of the test material that prevents the microorganism from growing over 10 minutes exposure but not at 5 minutes relative to that of phenol is considered as phenol coefficient of the test compound. In this example: PC= 320/160 PC= 2